Journal of
eISSN: 2473-0831


Review Article Volume 2 Issue 4
Correspondence: Prati Pal Singh, Centre of Infectious Disease, Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research, Sector-67, S. A. S. Nagar-160 062, Punjab, India, Tel +91-(0) 172-2214682-87, Fax +91-(0) 172-2214692
Received: May 10, 2016 | Published: June 1, 2016
Citation: Singh PP, Goyal A (2016) Macrophage-Mycobacteria Interaction: Exploration of Proteomic Signatures. J Anal Pharm Res 2(4): 00025. DOI: 10.15406/japlr.2016.02.00025
Tuberculosis (TB) has overburdened humans for ages and continues to be a major health problem worldwide. Mycobacterium tuberculosis, a facultative intracellular pathogen, is the causative agent of human TB. M. tuberculosis has an enormous capacity to survive and multiply inside host macrophages (MΦs), which are one of the most hostile cell types of the host. MΦs play a central role(s) as an effecter cell in host defense against mycobacterial infections. Soon after the inhalation of droplet-nuclei containing M. tuberculosis by the host, MΦs are the first cells to interact with mycobacteria, leading to the onset of primary events which later ensue in the development of TB. MΦs, all by themselves, are not sufficient to provide protective immunity; they require interaction with other cells (e. g. T-cells) to mount an enhanced protective immune response against the pathogen. The cytokines such as interferon gamma and tumor necrosis factor alpha etc. Play a major role in the regulation of interactions among different cells of the immune system to augment immunity. In this review, we focus on various functional roles of cytokines which serve as a link between innate and adaptive immunity, and discuss their potential use as biomarker(s) or biosignatures for the diagnosis, to monitor the progression of disease, and to determine the success of the treatment of human TB.
Keywords: biomarker, biosignatures, cytokines, macrophages, mycobacteria, proteomic signatures, tuberculosis
TB, tuberculosis; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; LJ, lowenstein-jenson; TLRs, toll like receptors; NOD, nucleotide oligomerization domain; IL, Inter Leukins; RNI, reactive nitrogen intermediates; DCs, dendritic cells; PGN, peptidoglycan; NO, nitric oxide; iNOS, nitric oxide synthase; KO, knock out; CCRs, chemokines receptors; IRFs, interferon regulatory factors; MIP, macrophage inflammatory protein; MCP, macrophage chemotactic protein; IP, inducible protein; GM, granulocyte macrophage; TST, tuberculin skin test
Tuberculosis (TB) is a global pandemic and a re-emerging disease caused by Mycobacterium tuberculosis which afflicts nearly one-third of humanity. Globally, in the year 2014, an estimated9.6 million new TB cases and 1.5 million deaths have been reported, and in the year 2015 global TB prevalence was 42 % lower than in 1999; mortality has fallen 47% since 1999.1,2 The Millennium Development Goal target to halt and reverse TB incidence has been achieved on a worldwide basis, and from 2016, the goal is to end the global TB epidemic by implementing the End TB Strategy.1 The world TB situation faces the formidable challenge due to the emergence of M. tuberculosis strains resistant to commonly used anti-TB drugs. Attention has now been focused on the burden of multi-drug resistant TB (MDR-TB; resistance to both the first-line anti-TB drugs isoniazid and rifampin)33 and the emergence of extensively drug resistant TB (XDR-TB); MDR-TB with additional resistance to any fluoroquinolone, and at least one of the three injectable second-line anti-TB drugs(capreomycin, kanamycin or amikacin) used in this treatment.4 The rise of drug-resistant M. tuberculosis strains in the presence of human immunodeficiency virus (HIV) is driving worldwide TB pandemic and in future, it is expected to turn from bad to worse.5,6
The principle event in the development of TB is considered to be the interaction(s) between MΦs and mycobacteria. Various virulence factors and host immune responses determine the fate of the disease. Virulence factors including mycobacterial cell wall components such as lipoarabinomannan (LAM) and acylated trehalose-2’-sulfate are known to be involved in the inactivation of mycobacterial phagosomes and the prevention of their fusion with lysosomes. Reportedly, the host immune response is of two types: innate and adaptive immune responses, and depends upon how these two types of immune responses coordinate to contain and combat TB. Various types of the cells of immune system and the soluble factors such as cytokines regulate the interaction between innate and adaptive immune responses.7 Several cytokines secreted by MΦs play important roles in protection against invading mycobacteria and the progression of mycobacteria-induced inflammation. How mycobacteria and MΦs interact with and utilize cytokines, still remains a mystery, largely due to the conflicting reports. The interaction of MΦs with different species of mycobacteria ensues in variations in the profile of elaborated cytokines. It is widely acknowledged that as of now, the diagnosis of TB heavily depends upon the century old techniques like acid-fast staining and Lowenstein-Jenson (LJ) slant culture etc., which in part, might be one of the reasons for rampant TB. Improper diagnosis not only affects the treatment of TB but also the discovery and development of new vaccines and drugs. Over the past few decades, the role(s) of cytokines has been evaluated in various pathological processes, which has convincingly shown the potential of cytokines to be used as biomarkers or biosignatures.
In this review, we spotlight on the role of cytokines, which correlates innate and adaptive immune responses. This review also emphasizes on the potential use of cytokines as biomarkers for the prognosis, diagnosis, etiology, disease severity, and development of new vaccines and drugs for the treatment of TB.
Immunopathology of M. tuberculosis
After this transition, there is a decrease in the number of blood vessels which penetrate the granuloma, and there is a noticeable increase in the number of foamy MΦs in fibrous capsule. Foamy MΦs are responsible for the accumulation of caseous debris at the center of granuloma, which signifies progression to the active disease. Caseous necrotic center of granuloma liquefies and cavitates under the condition of failing immunosurvillance, which is usually a consequence of malnutrition or/and HIV co-infection (fourth-stage). The necrosis of TB lesions has been correlated with bacterial burden, which indicates that with large burden, inflammation and damage is the usual outcome.11 Liquefied caseous foci provide an excellent opportunity for the extracellular growth of mycobacteria; the cavity formation also leads to the rupture of nearby bronchi, which allows thousands of mycobacteria to spread into the airways.12 All these processes lead to the development of productive cough which facilitates the generation of infectious droplets i.e. aerosol, and the completion of bacterial life cycle.
Recognition of mycobacteria
TLRs mediate the activation of various cells of innate immune system and several signal transduction pathways, which results in the destruction of invading bacilli. TLR signaling pathways are divergent and act either in a MyD-88-dependent or MyD-88-independent manner, leading to nuclear translocation of NF-κB (Figure 1). NF-κB is a transcription factor that is involved in the expression of several immune response genes such as those encoding TNF, interleukins (IL), co-stimulatory molecules and some effector molecules [reactive nitrogen intermediates (RNI), reactive oxygen species and defensins]15 all of which have direct antimicrobial activity. TLR signaling also triggers differentiation of monocytes into MΦs and dendritic cells (DCs), which play important role(s) in the expression of immune response. TLR family of receptors recognizes microbial components on the surface or within the extra compartment of cells (Figure 1). For the intracellular recognition of mycobacteria, a TLR-independent system is an essential requirement. The NOD families of proteins play an important role in the cellular recognition of mycobacteria. NOD1 and NOD2 are the archetypal members of the NOD-like receptor (NLR) family. Both NOD1- and NOD2-like receptors respond to a component of peptidoglycan (PGN). NOD1 is ubiquitously expressed and structurally related to apoptosis regulator Apaf-1. Human NOD1 activity is enhanced by γ-D-glutamyl-meso-diaminopimelic acid (γ-DAP)-containing muramyltripeptide. In terms of activity, human NOD1 is different from that of murine one, which recognizes DAP-containing muramyltetrapeptide.16 NOD2 recognizes muramyldipeptide and its expression is restricted to monocytes, MΦs, DCs and intestinal epithelial cells. Muramyldipeptide is found in all bacteria, whereas γ-DAP is restricted to gram-negative bacteria. Activation of NLRs by bacterial products can stimulate two major signaling pathways: a nuclear transcription factor pathway (as previously discussed) and an inflammasome pathway. NLR proteins activate caspase-1 with the help of inflammasome-complex proteins.17 A major function of inflammasome is to process the pro-inflammatory cytokine proIL-1β to its mature and active form (Figure 1). TLR and NLR signaling from different cellular compartments alerts the host to the impending immunological danger, and protects the host from inappropriate immunity generated in response(s) to mycobacteria.
Effector mechanism of MΦs to kill mycobacteria
Nitric oxide (NO): The inducible-nitric oxide synthase (iNOS) and nitric oxide (NO; non-specific, chemically reactive molecules) are the two most important molecules of innate immune system against mycobacterial infection in mouse model (Figure 1).18 Knock-out (KO) mice and immuno-deficient mice infected with M. tuberculosis are at a significantly higher risk of mortality. Activation of TLRs induces iNOS promoter activity which leads to the production of NO and killing of M. tuberculosis.19 iNOS molecules have been detected in MΦs from human disease lesions; however, it remains a matter of conjecture if NO is involved in protection against human M. tuberculosis infection. These observations also show the existence of NO-independent mechanisms but we considered it expedient to not to exclude the anti-microbicidal effects of NO.
Autophagy: Autophagy is an evolutionarily conserved fundamental cellular homeostatic process, which enables cells to clean up in a regulated manner. It is an unappreciated innate immune defense mechanism.20 It has been reported that lysosomal hydrolyzed ubiquitin peptides (Figure 1) have direct antimicrobial activity against M. tuberculosis.21 Several immune signaling molecules like IFN-γ, TNF, and CD40-CD40L interactions are known to enhance autophagy.22 However, autophagy is negatively regulated by IL-4 and IL-13. Studies involving autophagy as related to immune defense mechanisms have been better characterized in mouse system, than in human system. However, rapamycin and serum starvation stimulate autophagy-dependent antimicrobial activity in human MΦs, and shows the importance of autophagy in humans (Figure 1).
Vitamin D pathway: Vitamin D has been used to treat TB in the prebioticera. The role of vitamin D as an inducer of anti-microbial activity in human monocytes and MΦs has been reported.23 Vitamin D can be obtained from cod liver oil or synthesized in the skin during exposure to ultraviolet light. Vitamin D is readily metabolized in the liver to form 25 hydroxyvitamin D (25[OH]D), the accepted form of vitamin D. 1-α-hydroxylase enzyme Cyp27B1 converts 25[OH]D to a steroid hormone 1-α, 25-hydroxy vitamin D (1α,25[OH]2D), a biologically active metabolite. 1α, 25[OH]2D has been found to induce anti-mycobacterial activity in both monocytes and MΦs.23 1α, 25[OH]2D has been reported to bind the nuclear vitamin D receptor (Figure 1), which is already activated by signals from TLR, and enhances superoxide burst, phagolysosome fusion, induction of NOS and the activity of anti-microbial peptide (cathelicidin LL-37), which has been reported to exert anti-tubercular activity.24
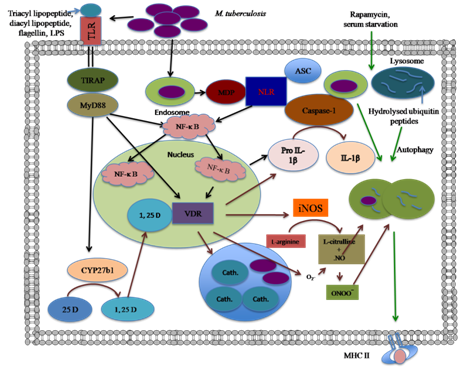
Figure 1 Different defense mechanisms of macrophages against intracellular M. tuberculosis; (1) Two different families of receptor for recognition of mycobacteria (shown in black arrow), (2) Vitamin D pathway (shown in brown arrow), (3) Autophagy (shown in green arrow).
Apoptosis: Cells encountered by mycobacteria sacrifice their life for the benefit of the remaining cells and it is termed as apoptosis i.e. programmed cell death. Several protozoan parasitic or bacterial pathogens have anti-apoptotic properties.25 Some reports have suggested that virulent M. tuberculosis induced host cell apoptosis,26 paradoxically, others have thought that it inhibited host cell apoptosis.27 Whether or not any particular mycobacterial strain inhibits apoptosis, is dependent upon the type of mycobacterial strain, differences in infection procedure, multiplicity-of-infection and, finally, the nature of host cell. The apoptosis can be induced via two major pathways: the intrinsic pathway, which is activated by intracellular stress, sensed by mitochondria and results in the activation of caspase-9, and the extrinsic pathway which involves death receptors like TNF-α receptor1 (TNFR1) and Fas/CD95, both of which activate caspase-8/10. Both these pathways lead to caspase-3/6/7 activation, which simplifies the subsequent steps, linked with apoptosis. Virulent mycobacteria cause up-regulation of anti-apoptic genes mcl-1 and A1,27,28 whereas similar studies with non-virulent mycobacteria do not cause up-regulation of these genes. Various studies carried out in KO mice have shown the importance of these genes in apoptotic inhibition. The virulent mycobacteria also decrease the expression of death receptors such as Fas/CD95 and soluble TNFR to inhibit apoptosis.
Cytokines: a link between innate and adaptive immunity: Cytokines are cell-secreted, low-molecular weight and highly potent glycoproteins through which cells communicate with each other. They are largely produced de novo upon stimulus (though a few of them are produced constitutively), and act as paracrine, endocrine and autocrine regulators at nano-molar or even at sub-nano molar concentrations. Cytokines may be classified as ILs (between leukocytes), lymphokines (produced by lymphocytes), monokines (produced by monocytes), chemokines (those responsible for chemotaxis) and growth factors (which stimulate proliferation, differentiation and the growth of specific cells) depending upon their main producer cell or function. Cytokines have major role(s) in homeostasis and inflammatory responses. Cytokines are vital component of immunity to TB, and play an important role(s) in innate and adaptive immune responses. Some of the important cytokines which function as a link between innate and adaptive immunity to TB are described below.
IL-12: IL-12 is a disulfide heterodimer molecule consisting of two subunits of unequal molecular weight of 35 kDa (p35) and 40 kDa (p40). Both these subunits together constitute p70, and are required for receptor binding and other biological activities of IL-12. The phagocytotic cells, after their infection with M. tuberculosis, are the major producers of IL-12 (Figure 2). IL-12 stimulates the growth of activated natural killer (NK) cells, T-cells and enhances the elaboration of IFN-γ, GM-CSF and TNF-α. IL-12 can also act as an endogenous adjuvant to boost immune response. Some studies have shown that administration of 32 kDa antigen of M. bovis BCG can enhance IL-10 and IL-12 production.29 Transgenic tomato expressing IL-12 has a therapeutic effect in a murine model of progressive pulmonary TB.30 Mutations in the gene encoding IL-12p40 and IL-12 R has been identified in non-tuberculous mycobacterial infections, and have shown impairment in mycobacterial immunity.31 Delivery of IL-12p40 results in the restoration of delayed-type hypersensitivity responses in IL-12p40-deficient mice infected with BCG,32 which shows its importance in immunity.
IL-23: IL-23, a heterodimer cytokine secreted by MΦs and DCs (Figure 2), is composed of a p40 subunit (shared with IL-12) and a separate p19 subunit unique to IL-27. The heterodimer receptor for IL-23 contains IL-12R1 chain and a unique IL-23R chain. IL-23 helps in the development of IL-17 producing T-cells, which enhance autoimmune inflammation. IL-23 is known to stimulate the secretion of IL-12 and IFN-γ by DCs in vitro, and has a proven role(s) in the activation of antigen-presenting cells.33 Some studies have reported a number of functions which were previously ascribed to IL-12, might belong to IL-23.34 The presence of p40 subunit alone has been reported to be sufficient for providing immunity against mycobacterial infections, which shows its major importance in immunity to TB.35 Chackerian et al.,36 have reported the role of IL-23 in reducing mycobacterial infection and in the promotion of granuloma formation in the absence of IL-12.36 IL-23p19-deficient mice, and mice treated with a specific anti-IL-23 p19 antibody did not show any granuloma formation in the presence of IL-12. Pulmonary IL-23 gene delivery increased the T-cell immunity and controlled the growth of M. tuberculosis in the lungs.37 IL-23could increase the Th1 and Th2 immune responses, when used as an adjuvant to DNA vaccines.38
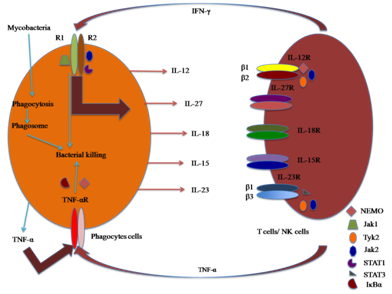
Figure 2 Diagram showing the role(s) of cytokines in association of innate (phagocytes cells) and adaptive immunity (T-cells).
IL-27: IL-27 is a new heterodimer cytokine which belongs to IL-12 family. It comprises of two subunits: Epstein-Barr virus-induced gene 3 (EBI3; an IL-12p40-related protein) and the other p28 (IL-12 p35-related polypeptide).39 Both EBI3 and p28 are up-regulated in the activated monocytes and DCs, which show that antigen-presenting cells are the main producer of IL-27 (Figure 2). TLLR (also known as WSX-1), a receptor, mainly expressed on naive CD4+ T-cells, available for binding of IL-27.39 The TLLR-deficient mice have been shown to have defect in Th1 response. It can be assessed by decrease in IFN-γ production, increase in IL-4 production, increased susceptibility to various infection and abnormal granuloma formation.40 The above mentioned account shows the importance of IL-27 in mounting Th1 response.
IL-15: IL-15 is a pleiotropic cytokine having a molecular weight of 14 kDa. Initially, in 1994, it was described as a T-cell activating factor, with structural similarity to IL-2.41 Mononuclear phagocytes and epithelial tissue cells mediate IL-15 production (Figure 2), in response to LPS, viral infection and external inflammatory signals. The heterodimer receptor for IL-15 contains β and γ subunits of IL-2 receptor and a high affinity binding α-chain of IL-15 receptor (IL-15Rα). IL-15 shares many common features with IL-2 due to the involvement of β- and γ-subunits of IL-2 receptor. Because of wide distribution of its receptor (IL-15Rα), IL-15 has been reported to stimulate NK cells, T-cells and also the cells of non-lymphoid organs.42 In the absence of IL-15 signaling, the population of CD8+ T-cells with memory phenotype gets significantly reduced.43 IFN-γ is known to be a potent inducer of IL-15. IL-15 administration has been reported to protect BALB/c mice against M. tuberculosis infection.44 All these observations demonstrate that IL-15 has a role(s) in host defense against M. tuberculosis infection.
IL-18: IL-18, a multifunctional pro-inflammatory cytokine is produced by a variety of cells including mononuclear phagocytes (Figure 2). IL-18 was originally described as an inducer of IFN-γ production by T-cells,45 but it alone is not responsible for IFN-γ production by T-lymphocytes. IL-18 requires the presence of secondary stimulus like IL-12 or microbial agents for IFN-γ production. IL-18 also stimulates the production of other pro-inflammatory cytokines, chemokines and transcription factors. So, it can be recognized as a cytokine which can potentially enhance both Th1 and Th2 responses.46 Several studies have shown the role(s) of IL-18 in host defense against a variety of pathogens including Salmonella typhimurium, Yersinia enterocolitica, Plasmodium berghei, Toxoplasma gondii, Leishmania and Herpes simplex virus type 1.47 IL-18 KO mice were highly susceptible to M. bovis BCG and M. tuberculosis infection,48 which clearly shows its protective role in mycobacterial infections.
TNF-α: TNF-α (a prototype pro-inflammatory cytokine), a 51 kDa homodimer, is secreted by infected monocytes, MΦs, DCs (Figure 3), T-cells, NK cells, and mast cells. Two distinct receptors for TNF-α are encoded by separate genes. TNF-α contributes its major role in the regulation of apoptosis of M. tuberculosis-infected cells (Figure 3).49 Apoptosis, in turn, could contribute to protection either by direct killing of M. tuberculosis or by presenting the antigen by DCs. Maturation of DCs was promoted by TNF-α (Figure 3), thereby inducing migration and up-regulation of the molecular pattern required for activation of T-cells.50 TNF-α in association with IFN-γ has been reported to activate murine MΦs for the killing of intracellular M. tuberculosis via the induction of RNI (Figure 3),51 whereas TNF-α-deficient mice have been reported to produce insufficient amount of RNI, and thus fail to control the disease progression.52 In some in vitro experiments, TNF-α has been reported to support the growth of virulent mycobacteria, which create doubt about its role(s) in the induction of nitrogen radicals.53 TNF-α has a major role in granuloma formation, and also has some immune-regulatory properties.54 Increased susceptibility to mycobacterial infection was observed in TNF-α or TNFR-αKO mice.55 Another major function of TNF-α is to recruit T-lymphocytes and monocytes to the site of infection, and the movement of these cells occurs because of its action on vascular endothelium (Figure 3).56 TNF-αdecreased the expression of chemokine receptors (CCRs) such as CCRs and induced the production of regulated on activation normal T-cells expressed and “(RANTES)”.57 The role of TNF-α, therefore, is like a ‘double-edge sword’ because it not only helps in granuloma formation but also cause host-mediated destruction of lung tissue. The neutralization TNF-α results in increased risk of TB, increased production of IL-12 enhanced expression of chemokines and decreased IFN-γ production in blood. Patel et al.,58 have shown that HIV-infected individuals have impaired TNF-α-mediated MΦ apoptotic response to M. tuberculosis.58 No association between gene polymorphism of TNF-α and disease susceptibility due to M. tuberculosis infection has been established, yet.
IFN-γ: IFN-γ, a highly pleiotropic protein of 34 kDa can promote host defense and immunopathologic processes. Activated T-cells, MΦs and NK cells are the major producers of IFN-γ. All nucleated cells have receptor for the recognition of IFN-γ. IFN- receptors consist of a larger subunit (IFNGR-1) and a smaller subunit (IFNGR-2). The studies of the mechanism of action of IFN-γ have led to the discovery of Jak-Stat signaling pathway. IFN-γ regulates an extensive number of genes. One of the most important gene; interferon regulatory factors (IRFs) bind to myeloid differentiation factor 88 (MyD88) and migrate to nucleus for up-regulation of gene expression. Importance of MyD88 dependent IRF-1 activation was demonstrated by increased susceptibility to M. tuberculosis infection in mice deficient either in MyD88 or IRF-1. The other genes, which are up-regulated include iNOS and NADPH-dependent phagocyte oxidase both of which can help provide resistance to mycobacteria.59,60 The other application includes up-regulation of ATP receptor (P2X7), TNFR and TLR2, which enhance the pro-inflammatory response of MΦs.61 The IFN-γ-induced activation of MΦs enhanced pinocytosis, receptor-mediated phagocytosis and the killing of M. tuberculosis. IL-12 secreted by activated MΦs has been reported to induce the release of IFN-γ by antigen stimulated T-cells (Figure 2), whereas IFN-γ, in turn, has been shown to induce augmented secretion of IL-12 by the activated MΦs.62 Both IL-4 and IL-10 are known to suppress the production of IFN-γ by CD4+ T-cells. IFN-γ gene transfer in severe combined immunodeficiency mice resulted in enhanced bactericidal activity of MΦs.63 On the basis of these reports, we can infer that IFN-γ can be used as an adjuvant cytokine for the control of drug-resistant M. tuberculosis, and can used to shorten the duration of anti-TB treatment.
Anti-inflammatory cytokines
IL-10: IL-10, an 18 kDa potent anti-inflammatory cytokine is produced by activated MΦs, lymphocytes and some non-lymphocytic cell types (e.g. keratinocytes). Receptors for IL-10 belong to two cysteine/WSXWS family and the signal transference occurs with the help of Jak/STAT pathway.64 The major function of IL-10 is to decrease the production of RNI, Th1 cytokines and reduced expression of class II MHC molecules.65 Transgenic mice which over-express IL-10 have been reported to be more susceptible to mycobacterial infection as compared to the wild-type ones.66 IL-10 or IL-10 receptor-blocking antibodies are known to inhibit the multiplication of M. avium.67 M. tuberculosis infection in C57BL/6 or the BALB/c miceensued in differential elaboration of IL-10, and M. tuberculosis-infected BALB/c mice showed higher levels of serum IL-10 as compared to C57BL/6 mice. IL-10 also promotes the disease progression in CBA/J mice, and thus can be expected to be used as a potential biomarker of TB progression in humans.68 Shin et al. have shown that one common promoter SNP in IL-10 decreased the risk of TB.69 In anergic TB patients, IL-10 production was higher both before and after the treatment of TB which suggests its role in causing the suppression of an effective immune response.70
TGF-β: TGF-β is a 25-kDa polypeptide and seems to counteract protective immunity in TB. It exists in three isotype forms known as TGF-β1, TGF-β2 and TGF-β3. Lipoarabinomannan and immunogenic surface of mycobacteria induce the production of TGF-β by monocytes and DCs. TGF-β is highly expressed at the site of the disease, is produced in higher amounts, and is believed to be involved in some immune regulatory activities. The action of TGF-β is highly pleiotropic. It inhibits the growth of some cell types and stimulates the growth of the other cell types. TGF-β suppresses the cell-mediated immune response at multiple levels like the inhibition of the expression of IFN-γ receptors and thus hinders the production of cytokines. Abnormalities like tissue damage, fibrosis, collagen synthesis which are generally observed during TB, may be caused by TGF-β.71 TGF-β, in association with IL-10, is known to suppress the production of IFN-γ.72 Hernandez et al.,73 have suggested that a combination of TGF antagonist and the inhibitors of cyclooxygenase provide an effective alternative treatment for TB.73
IL-4: IL-4, a prototypeimmuno-regulatory lymphokine, is a 20 kDa glycoprotein with 129 amino acids that is secreted by T-cells, mast cells and basophils. It stimulates cells of hematopoietic and non-hematopoietic origin, and causes the induction of Th2 response. Its deleterious effect includes suppression of cell-mediated immunity, tissue damage and fibrosis.74 The function(s) of monocytes may be enhanced by IL-4, which causes an increase in the expression of MHC-II, CD23 and chemokines.75 IL-4 and T-cells are required for the generation of IgG1 antibodies against cardiolipin. Some observations have shown that increased IL-4 expression in lymphocytes promotes CD30 expression, which, in turn, further reduces the expression of TNFR-associated factor 2 and may account for the TNF-mediated apoptosis.76 Recent studies in IL-4 KO, M. tuberculosis-infected mice have shown an increase in the mycobacterial outgrowth and the size of granuloma.77 The increased production of IL-4 has also been reported to be associated with the reactivation of latent TB infection, and the intensification of tissue damage in experimental active TB infection.78 Thus, the role of IL-4, whether or not it helps in the progression of TB or just acts as a marker of the disease progression, still remains unanswered.
Chemokines
Chemokines (chemo-tactic cytokines) are a group of structurally-related polypeptides with molecular weight between 8-13 kDa. Chemokines can be distinguished from other cytokine molecules in that they are the only cytokines which act through the interaction with a specific subset of seven transmembrane domains and G1 protein-coupled receptors.79 The human chemokines system comprises of more than 50 chemokines and 18 different types of chemokine receptor. Chemokines can be divided in to four families according to N-terminal cysteine positioning in their amino acid sequence (CXC or alpha, CC or beta, CX3C or delta, C or gamma subtypes) with L for ligand and also divided into two categories on the basis of their physiological features (constitutive or inducible). Out of the 50 chemokines, a majority of them can be categorized as CXC or CC chemokines. Chemokines induce the change in cell architecture and cell membrane protein composition, thus enhancing the penetration of leukocytes through the vascular endothelium into the inflamed tissues.80 Because of this ability of chemokines, they represent important mediators of innate immune response against infection. CXC subfamily contains IL-8, protein KC, macrophage inflammatory protein (MIP-2), interferon-inducible protein (IP-10) and CXCL9 (MIG), whereas C-C subfamily contains macrophage-chemotactic protein (MCP-1), MIP-1α/β, RANTES, MCP-3 and MCP-5. CC type chemokines activate and attract monocytes, MΦs, lymphocytes, NK cells, eosinophils, basophils and DCs,81 whereas CXC chemokines attract and activate neutrophils, T-cells and NK cells. Receptors bind to various classes of chemokines and based on this, it has been named CXCR1 to CXCR5 (bind the CXC chemokines); CCR to CCR9 (bind the C-C chemokines); XCR1 (bind the C chemokines); CX3CR1 (binds the CX3C chemokines; R stands for receptor). Currently, 18 human chemokines receptor are known which include 6 CXRs, 10 CCRs, one XCR, and one CX3CR. The structure of chemokinesincludes monomeric fold, 3β strands, ε erminal helix and flexible N-terminal region. This flexible N-terminal region is important for receptor activation. CCL1, CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES) are produced by human MΦs following their infection with virulent strains of M. tuberculosis, whereas CXCL8 (IL-8) and CXCL1 are produced by M. tuberculosis-infected polymorphonuclear granulocytes.82 In response to virulent Erdman M. tuberculosis, MΦs produced different types of chemokines like CCL4, CCL5 and CXCL9 (MIG). Similarly, several other chemokines like CCL2 (MCP-1), CCL3, CCL7 (MCP3), CCL12 (MCP5), CXCL2 (MIP2) and CXCL10 (IP-10) were also produced in response to M. tuberculosis. All these data show that mycobacteria are strong inducers of chemokines, and different strains of mycobacteria induce the elaboration of chemokines in a differential manner.
MCP-1, an 8.7 kDa protein, is a product of an early competence gene JE, and a potent chemoattractant for both activated T-lymphocytes and monocytes. Some researchers have observed that the functional promoter polymorphism in MCP-1 is associated with increased susceptibility to TB.83 Recently, it has been demonstrated that exogenous administration of MCP-1 results in the production of superoxide anion, which shows that MCP-1 can be used as an immune prophylactic agent.84 RANTES (CCR-5) is involved in recruitment of T cells to inflammatory site, activation of T-cells and inhibition of the intracellular growth of mycobacterium.85 In a murine model, some correlation has been observed between the development of M. bovis-induced granuloma formation and RANTES.86 MIP-1α/β(a low molecular weight heparin-binding protein) mainly attracts CD8+ T-cells, whereas MIP-1β mainly attracts CD4+ T-cells. Induced chemokines (like MIP-1α can modulate MIP-1β and induce TNF-α secretion) can act in an autocrine manner and regulate various MΦ responses.87 CXCL-8, a most potent activating factor and attractant for lymphocytes, enhances non-oxidative intracellular killing of mycobacteria inducing degranulation, respiratory burst and change in phagocytic activity. The presence of CXCL-8 reduced the survival of M. tuberculosis in MΦs; paradoxically, its absence increased the survival.88 IP-10, a 10 kDa protein, an unusual member of α subfamily, activates T-lymphocyte, monocytes, and may be involved in delayed-type hypersensitivity. A diagnostic test for the detection of M. tuberculosis using IP-10 was performed in both active and latent TB, and the resultshave shown its utility as a biomarker.89 IFN-γ has been shown to play an important role in the regulation of the expression of CXCL-10 during M. bovis BCG infection. CCL7 (MCP-3) mainly attracts monocytes and activates a variety of inflammatory cells. One report has shown increased MCP-3 concentration in biopsy specimens of subjects with pulmonary TB [90]. Both chemokine(s) and theirreceptor(s) show redundancy in their expression, because a particular chemokine can bind to more than one receptor, and most of the receptors can bind to more than one cytokines. The lack of the complete understanding of the role(s) of each chemokine may be the reason for redundancy in expression of these chemokines. As per prior knowledge, no clear change in the profile of chemokines in TB patientshas been identified yet.
Colony-stimulating factors (CSFs)
CSFs are a group of glycoproteins of 18-90 kDa, and play pleiotropic roles in the stimulationof the generation, differentiation, and proliferation of myeloid hemopoietic progenitor cells into MΦs and neutrophils.91 Four different known types of CSFs are granulocytes (G)-CSF, macrophage (M)-CSF, granulocyte-macrophage (GM)-CSF and multi-CSF (IL-3). Both G-CSF and M-CSF are lineage specific, and thus stimulate G and M colony formation. GM-CSF is known to induce colony formation in eosinophil’s, G and M cells. Multi-CSF stimulates formation of colonies which contains cells of various lineages. Structurally, there is not much difference among the members of CSFs; however, still M-CSF is heavily glycosylated, and disulphide-linked homodimer, whereas G-CSF, GM-CSF and multi-CSF consists of a single polypeptide chain. MΦs, endothelial cells, type II alveolar epithelial cells and activated T-cells release CSFs in response to pro-inflammatory cytokines. CSFs enhance immmuno-stimulatory activities of DCs by increasing the surface expression of MHC II and co-stimulatory molecules.92 GM-CSF-deficient mice are unable to mount an effective immune response against M. tuberculosis infection. Co-immunization using CSFs enhance the expression of antigen specific IFN-γ secreting T-cells and contributes to protection.93 CSF-deficient mice have decreased number of alveolar MΦs and show an increased susceptibility to infections.94 Antimicrobial activity of human MΦs can be increased by their treatment with recombinant CSFs.95 Vaccine-encoded GM-CSF causes modulation in DC functions, and enhancement in protective immunity against M. tuberculosis infection.96 Previous studies have reported that purified mouse SAP can induce the production of serum CSFs in mice and, which in turn can induce LPS-independent de novo production of CSFs by elicited macrophages, in vitro.97 Our lab has earlier reported that a 30-kDa secretory protein of M. tuberculosis H37Rv also induced the production of CSFs.98
Biomarkers
A biomarker is defined as a characteristic that is measured and assessed as an indicator of normal biological processes, pathogenic processes or pharmacological responses to therapeutic interventions.99 It may be any factor whose value is consistently associated with changing health or disease states. In clinical trials, biomarkers may form a basis of a surrogate endpoint that can substitute for a clinical end-point, based on epidemiological, therapeutic, pathophysiological or other scientific evidences. The valuable applications of the biomarkers include the following:
Drug development: During drug development, biomarkers can assist in target selection and lead identification, optimization, demonstration of the proof of concept, selection of appropriate dose, dosing schedule, and selective drug combinations with additive or synergistic interactions.100
Novel vaccine development: Albert Calmette and Camile Guerin successfully developed first vaccine bacilli Calmette-Guérin (BCG), against TB. This vaccine protects the infants from TB but has no effect on TB in adults. Therefore, novel vaccines are desperately required. Biomarkers help in predicting safety and vaccine efficacy at an early stage that would effectively shorten the duration of clinical trials, hence quite useful in novel vaccine development.101
Clinical care: TB patients face long-term chemotherapy with multiple drugs to avoid disease reactivation and prevent drug resistance, which poses a major challenge in the eradication of TB. Both emergence of drug resistant strains and their dissemination can be reduced by short term of drug therapy. Biomarkers that predict successful pathogen eradication would allow shortening of the duration of chemotherapy in an individualized manner.
Co-infection: Emergence of acquired immunodeficiency syndrome has become a driving force for the re-emergence of TB. These co-infected patients are more vulnerable to disease because of the impaired T-cell functions.102 Biomarkers help in predicting the need for preventive therapy against TB, and it could reduce the spread of TB due to HIV co-infection.
Childhood TB diagnosis: Diagnosis of TB largely depends upon chest X-ray, smear microscopy and tuberculin skin test (TST) in adults, and these tests are more complicated in children. Due to BCG vaccination or infection by mycobacteria other than TB (MOTT) in children, they give false positive or negative results during TST test. Here the cytokines as biomarkers would be a perfect answer to the dire need of having molecules of diagnostic utility to exquisitely distinguish between TB and MOTT.
Cytokines as biomarkers: The utility of cytokines as biomarker is very fascinating because of their direct role in immunity to M. tuberculosis. Cytokine expression changes in early infection, long before clinical or bacteriological changes, and thus can be expected to function as potential biomarkers. Cytokines logically seem to be good candidates for surrogate endpoints to predict clinical outcome of the disease based on epidemiologic, therapeutic, pathophysiological and other scientific evidences. The role of IFN-γ in protection against mycobacterial infection, and increased expression of IFN-γ in TB patients who successfully complete therapy, as well as in healthy and latently infected individuals suggests that it might act as a good biomarker.103 The development of in vitro assays which measure the production of IFN-γ in response to M. tuberculosis-specific antigens has been the major break-through inthe diagnosis of TB.104 IFN-γ release assays (IGRAs) such as Quanti FERON TB Gold (Cellestis, Carnegie, Australia) and T-SPOT TB (Oxford immunotech, Oxon, UK) are highly specific, sensitive, and the results can be obtained overnight. Some current evidences suggest that IGRAs tests have low false +ve as compared to TST. The performance of IGRAs is still questionable in HIV co-infected individuals and in TB endemic countries. Therefore, analysis of IFN-γ alone is not sufficient as a biomarker. So, there is need for the identification of other cytokines which can function as biomarkers, in future. Alterations in IFN-γ responses in TB patients are associated with the alteration in other cytokines. In the mouse infection model of TB, Darah et al.,105 have shown that the degree of protection induced by BCG is predictable by the frequency of CD4+ T-cells which produce IL-2, TNF-α, and IFN-γ.105
GM-CSF levels, in contrast to other cytokines, were increased in children with active TB as compared to the healthy children with latent M. tuberculosis infection and, thus it can be used as a biomarker. in vitro and in some mouse models of TB, treatment with recombinant GM-CSF results in significant decrease in the number of viable mycobacteria in MΦs, blood, liver and spleen. An open trial with a daily dose of IL-2injection, subcutaneously, was set up during the first month of a conventional multi-drug anti-TB therapy. Under these conditions IL-2 was found to be safe, and had significant additive effects on the chemotherapy of TB.106 An additional study has shown that recombinant human IL-2 adjunctive therapy has favorable clinical effects on patients with MDR-TB.107 Expression of IL-4 mRNA is elevated in healthy TB contacts that later develop the diseases, and its level decreases in treated TB patients, as IFN-γ level increases in these patients.108 The IL-4 antagonistic splice variant, IL-4δ2, behaves similarly to IFN-γ; the ratio of IL-4δ2 to IL-4 increased overtime in patients recovering from TB. IL-4δ2 mRNA levels were also elevated in individuals with latent TB, who presumably control reactivation of pathogen by making a protective immune response. Elevated levels of TNF-α have been proposed as a biomarker in TB negative smears. IP-10 and MCP-2, which are expressed in inflamed tissues by resident and infiltrated cells (primarily monocytes, MΦs) show their diagnostic potential, and thus can be used as biomarkers. Monocytes and MΦs secrete chemokines after paracrine stimulation from T-cells by IFN- and other pro-inflammatory cytokines or through innate mechanism.109,110 IL-1 receptor antagonist (IL-1RA) is a natural antagonist of IL-1 produced by MΦs. IL-1RA shows its response in TB treatment, and has been suggested as a marker of TB.111 The levels of CCL-2, which primarily targets monocytes and T-cells, have been associated with TB and its treatment response. CCL-7 levels have been found to be elevated in bronchoalveolar lavage fluid and biopsy specimens of subjects with TB. Hence analysis of different cytokines is a promising tool for predicting protective immunity against M. tuberculosis.
In today’s world, there is an ever increasing demand for new and improved point-of-care and biomarker-based diagnostic tests and tools for TB, which have higher detection rates, high specificity and sensitivity, are easy to perform, and affordable by those in need. Several new technologies for the diagnosis of TB have been developed but no major break-through has been achieved so far. Nevertheless, it is now well established that cytokines (though less high-throughput-oriented) are one of the most important proteomic signatures and biological markers (biomarkers) of infection, disease and protection/treatment outcome of TB.112-114 Undoubtedly, it is especially so, because of the specific, intense and intimate interaction between MΦs and M. tuberculosis, the most important step in the of the pathogenesis of TB, which invariably ensues in the elaboration of an array of cytokines, the permutation and combination of which, is believed to better reflect the state of the disease at a particular point in time, as compared to the other protein and non-protein components of M. tuberculosis (which are not the product of the host cell and pathogen interaction) or only the host components. Additionally and more pointedly, cytokines and chemokines have been reported to have major role(s) in the determination of the progression and the outcome of M. tuberculosis infection in humans and animals.115,116 However, surprisingly, little attention has been paid to the interdependency of cytokine levels in their potential applications as biomarkers. So far only a few reports have pointed out that certain pathological situations can be best evaluated by considering the ratio of two or more different cytokines, which may just be a reflection of the complex relationship between several cytokines which act during a particular pathological event. These relative ratios of two or more different cytokines may turn out to be the most important and reliable diagnostic biomarkers than the absolute concentration of individual cytokines, which tend to show important inter and intra-individual variability, depending on gender, age, and life style. Once a mass data has been generated under optimally standardized conditions and with different variables well tested, documented and accounted, the knowledge so generated may be used in the commercial development of biomarker-based kits for the diagnosis of TB, in which cytokines will surely be included. By using cytokines as biomarkers, drug tolerance and resistance due to suboptimal treatment would be minimized and, clinical trials of novel anti-TB drugs and vaccine candidates will be accelerated and shortened. Presently, there is growing global interest and investment in finding more and better biomarker(s) for this ancient scourge of humanity. Nevertheless, there are big challenges, lots of hope and, good opportunities.
We are grateful to Dr. K. K. Bhutani, Officiating Director, National Institute of Pharmaceutical Education and Research, for his help and continued encouragement. Mr. Amit Goyal is grateful to the Council of Scientific and Industrial Research, New Delhi, India, for the award of a Senior Research Fellowship (National Entrance Test). This is NIPER communication No. 497.
Authors declare that there is no conflict of interest.

©2016 Singh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.