Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 3
Correspondence: Shaukat Ali, BASF Corporation, 100 Park Avenue, Florham Park, NJ 07932, USA, Tel 973-245-6506
Received: April 22, 2016 | Published: May 10, 2016
Citation: Fussnegger B, Tawde V, Chivate A, Kolter K, Ali S (2016) Kollicoat® IR: Minimizing the Risks for Oxidative Degradation of Drugs. J Anal Pharm Res 2(3): 00020. DOI: 10.15406/japlr.2016.02.00020
Kollicoat® IR, a graft copolymer comprised of polyethylene glycol and polyvinyl alcohol (PEG: PVA, 1:3), has been used as an instant release coating of tablets and pellets. The aim of this study was to investigate the peroxide free instant release polymer in the instant release matrix tablet and examine the oxidative degradation of raloxifene when used as a wet binder. The data suggests that Kollicoat® IR prevents the formation of raloxifene N-oxide, an oxidative adduct formed by oxidation of reactive tertiary (3°) amine moiety. As compared to other binders such as Povidone K29/32 and K30, the oxidative degradation was imminent caused by the presence of peroxides in these ingredients. If the peroxides were controlled by anti-oxidant such as Kollidon® 30 LP or by packaging such as the Kollidon® 30 in smart packaging (or PeroXeal®), the oxidative degradation was minimized and aligned with the data as observed with Kollicoat® IR. This study clearly demonstrates that Kollicoat® IR lacked the peroxides which in turn reduced the formation of raloxifene N-oxide.
Keywords: Kollicoat® IR, graft copolymer, PEG-PVA, binder, raloxifene, peroxides, oxidative degradation
Binders are important ingredients of solid oral dosage formulations (SODFs). The structure and function of binders play an important role in dosages’ shape and size, delivery, and stability of drug products. The stability of active ingredients depends on external factors, e.g. exposure to heat, oxygen and humidity but also on internal factors, e.g. the impurities such as peroxides, aldehydes, heavy metals and moisture contents often associated with the excipients.1,2 These impurities may lead to undesired reactions and alter the efficacy of dosages with possibly adverse effects.3 Thus, controlling these impurities is important in alleviating the degradants to enhance the shelf life of drug products. Peroxides, amongst many of the impurities, remain the most challenging in drug development. For instance, wet granulation though remains widely practiced in the industry for its simplicity and easy scale up, can exert an enormous mechanical stress on the excipients caused by multiple formulation steps involving blending, mixing, granulation, drying, and sieving.4 Such processing steps can lead to elevated impurity levels. Thus, minimizing the risks for elevated impurities in the excipients, especially the peroxides, is highly essential, which could otherwise be detrimental to long term stability of pharmaceutical dosages.5,6
Oxidative degradation has been studied extensively.7-9 In a matrix dosage wherein the drugs and excipients typically are intimately in contact, an elevated level of peroxide may lead to significant oxidation of sensitive drugs, especially those bearing tertiary amines and secondary alcohols. Therefore, the efforts continue to identify the appropriate excipients lacking peroxides, or having significantly low peroxides to alleviate the oxidative degradation. This article describes the use of Kollicoat® IR, a grafted copolymer comprised of polyethylene glycol and polyvinyl alcohol (PEG-PVA), as a wet binder in tablets. PEG-PVA, developed first as an instant release coating polymer for immediate release coatings, has also been used as a hydrophilic pore former in the drug layering for sustained release tablets.10
This study aims at examining the binding property of Kollicoat® IR, which is referred as PEG-PVA, as a “peroxide free” binder for drugs sensitive to oxidative degradation. Raloxifene, bearing a tertiary amine and being highly sensitive to oxidation, has been investigated to demonstrate the feasibility of PEG-PVA as an alternative binder to control oxidative degradation to N-oxide, and perhaps other sensitive drugs alike.
Kollicoat® IR, Kollidon® 30, Kollidon® 30 LP, Kollidon® CL, Kollidon® VA64, ascorbic acid, were obtained from BASF, Germany, Plasdone® K29/32 from Ashland, USA, raloxifene hydrochloride was obtained from Aarti Drugs Ltd, Mumbai, India, Aerosil® 200 from Evonik, Germany, HPMC 3 mPas (Pharmacoat 603) and HPMC 6 mPas (Pharmacoat 606 were purchased from Shin-Etsu, Japan, corn starch was from Cerestar, Belgium, lactose monohydrate (Sachelac 80) from Meggle, Germany, microcrystalline cellulose (Avicel PH 101) used was from FMC, USA, magnesium stearate was obtained from (Bärlocher, Germany) Fluid bed granulation was carried out on GlattGPC G3 (Germany).The ascorbic tablets were compressed on a Korsch PH 106 (Germany) rotary press equipped with Compression Research System., whereas, the raloxifene tablets were compressed into tablets on a 8 stations rotary press, India. Unless noted otherwise, the statistical analysis was not performed as all the data points were a single measurement on each individual samples.
Kollicoat IR® (PEG-PVA): structure and properties
PEG-PVA is highly flexible polymer and the inherent flexibility of films is due to the presence of PEG covalently linked to PVA as shown in Figure 1 & Table 1.11
|
Property |
PEG-PVA |
HPMC 2910 Type 3 |
HPMC2910 Type 6 |
|
Viscosity at 20 wt% (water) |
115 mPas |
800 mPas |
5150 mPas |
|
Oxygen permeability (cm3/100 µ/m2.d) |
104 |
700 |
640 |
|
Water vapor permeability (cm3/100 µ/ m2.d) |
510 |
400 |
500 |
|
Elongation at break, film thickness 100 µ |
113 % @ 33% RH105% @ 54% RH 70% @ 75% RH |
4-5% @ 33% -75% RH, and 11-17% @ 33%-75% RH |
n.d. |
|
Disintegration time, film thickness 100 µ |
0:44 sec @ pH 1.2, pH 6.8 |
1:30 min @ pH 1.2, pH 6.8 |
3 min @ pH 1.2, pH 6.8 |
|
Dissolution time, film thickness 100 µ |
1:30 min @ pH 1.2, pH 6.8 |
4-5 min @ pH 1.2, pH 6.8 |
6:30 min @ pH 1.2, pH 6.8 |
|
Surface tension, 23 °C, 10% |
44 mN/m2 |
47 mN/m2 |
n.d. |
Table 1 Physico-chemical properties of PEG-PVA copolymer
In comparing with cellulosic excipients, the PEG-PVA with lower viscosity provides a better alternative to HPMC in granulation and spray coating, which reduces time and saves cost. In addition, PEG covalently bound to polyvinyl alcohol acts as an internal plasticizer and provides a high flexibility, thus allowing the polymer to overcome the mechanical stress during manufacturing and storage of dosage forms.
Wet binder in formulation of ascorbic acid tablet
The properties of PEG-PVA as a binder have been evaluated in wet and fluid bed granulations.12-14 In wet granulation, for instance, the placebo granules comprised of lactose monohydrate, MCC, crospovidone (Kollidon® CL) were prepared with 3%, 4%, and 5% PEG-PVA binder solutions, and compared with povidone K30 (Kollidon® 30) with the corresponding amounts. Tablet properties such as tablet weight, thickness, hardness, friability, and disintegration time with PEG-PVA and povidoneK30 were evaluated and found to be comparable with both binders with the individual corresponding amounts used. In fluid bed granulation, the PEG-PVA was used with ascorbic acid. The granules were also prepared with copovidone (Kollidon® VA64) and HPMC with 7% binder solution under the similar conditions as PEG-PVA. Granule properties such as particle size and compression profile were evaluated, and compared with copovidone and HMPC granules. The granules with high shear mixing with 15% binder solution were also prepared with PEG-PVA, copovidone and HPMC and the compression profiles were also measured and compared with fluid bed coated granules. Figure 2, illustrates the compression profiles of granules prepared by high shear and fluid bed granulations with PEG-PVA, copovidone and HPMC polymers. The hardness of the granules, increased as a function of compression forces in both fluid bed and high shear granulations. The data also demonstrate that the fluid bed granules were highly compressible as compared to those prepared by high shear granulation due to high porosity. In the high shear mixing, the granules however were densely packed, less porous, and hence were less compressible. Taken collectively, the data suggest that PEG-PVA exceptionally performed as a wet binder in fluid bed and high shear granulations, and the compression profiles of resulting granules were similar to those obtained with copovidone, HPMC and povidone K30.
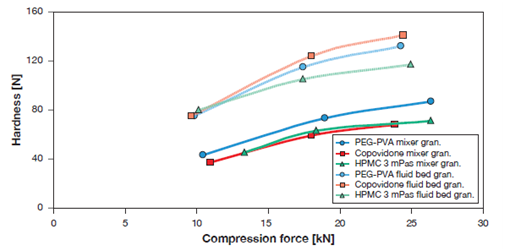
Figure 2 Compression profiles of ascorbic acid tablets prepared from the granules prepared by fluid bed and high shear granulations; the compressions were carried out on each individual samples, hence no statistical data.
Stability study of PEG-PVA
Table 2 shows the peroxide levels of PEG-PVA under stability conditions. The data demonstrate that the peroxide contents remained unchanged under all stability conditions examined, suggesting that the PEG-PVA is free of peroxides at 25°C/60% RH, 30°C/35% RH and 30°C/70% RH over 5 years, and also under the accelerated conditions 40°C/75% RH for over 18 months.
|
Stability condition |
3 mo. |
6 mo. |
12 mo. |
18 mo. |
24 mo. |
36 mo. |
48 mo. |
60 mo. |
|
25°C/60% RH |
< 1 |
< 1 |
4 |
3 |
< 1 |
< 1 |
< 1 |
< 1 |
|
30°C/35% RH |
< 1 |
< 1 |
2 |
- |
- |
- |
- |
- |
|
30°C/70% RH |
< 1 |
< 1 |
1 |
- |
< 1 |
< 1 |
< 1 |
< 1 |
|
40°C/75% RH |
< 1 |
< 1 |
1 |
1 |
- |
- |
- |
- |
Table 2 Stability of PEG-PVA to monitor the peroxides (denoted in mEq/kg*).1
*mEq/kg is equivalent to 17 ppm (based on 0.5 of the molecular weight of hydrogen peroxide)
PEG-PVA casted films (100 microns) were evaluated under the accelerated conditions at 40 °C/75% RH for 3, 6, 12 and 18 months, but the peroxides remained at <1 mEq/kg. The data clearly suggests that the peroxides did not increase over the entire span of stability study at ambient and accelerate conditions. The values of peroxides were typically below <17 ppm levels from single measurement on each individual sample.
PEG-PVA: An enabler for peroxide sensitive APIs
PEG-PVA as a binder was examined with raloxifene, a highly peroxide sensitive drug in the tablet formulation with a loading of 30%,and compared with povidone K29/32 (Plasdone® K29/32) and povidone K30 (Kollidon® 30) binders,each containing different amounts of peroxides. Table 3 shows the binders with different peroxide levels and Table 4 shows the raloxifene tablet compositions prepared with the binder solutions at 3% and 6%. Evidently, PEG-PVA had the peroxides <17 ppm, while the other binders with regular packaging contained >200 ppm, but the povidone in EVOH (PeroXeal®) packaging contained about 100 ppm, and the povidone K30 LP contained 30 ppm of peroxides.
|
Binder |
Peroxides, ppm* |
|
PEG-PVA |
<17 |
|
PVP K30 LP (PeroXeal® packaging) |
30 |
|
PVP K30 (PeroXeal packaging) |
98 |
|
PVP K30 (PE in-liner) # 1 |
333 |
|
PVP K30 (PE in-liner) # 2 |
233 |
|
PVP K29/32 |
305 |
Table 3 Binders and their peroxide levels in raloxifene tablets
|
Ingredient |
Binder @3% |
Binder @6% |
||
|
Amount (mg/tablet) |
% |
Amount (mg/tablet) |
% |
|
|
Raloxifene HCl |
60 |
30 |
60 |
30 |
|
Dicalcium Phosphate |
128 |
64 |
122 |
61 |
|
Binder |
6 |
3 |
12 |
6 |
|
Aerosil® 200 |
4 |
2 |
4 |
2 |
|
Mg Stearate |
2 |
1 |
2 |
1 |
|
Total |
200 |
100 |
200 |
100 |
Table 4 Composition of raloxifene tablets
*Tablets were compressed at 9 kN and the hardness of tablets ranged between 14 and 18 kP, with the friability <0.05%. The moisture content in the granules was about 1% regardless of the binders used.
Stability of raloxifene tablets
The oxidative degradation of raloxifene to N-oxide is shown in Figure 3. The formation of N-oxide was monitored during the stability study of raloxifene tablets carried out under the accelerated conditions of 40°C/75% RH for period of 1, 3 and 6 months, and the data are shown in Table 3, 4.
The stability data, as shown in Table 5, suggests that raloxifene tablets with PEG-PVA were stable over 6 month period without degradation. Likewise, the tablets with povidone K30 bearing the low peroxide (e.g. Kollidon® 30 LP) and the smart seal (PeroXeal®) povidone K30, showed similar results to those with PEG-PVA, suggesting no evidence of oxidative degradation of raloxifene. In contrast, povidone K30 with PE in-liner packaging and povidone K29/32 with peroxides >200 ppm, showed an appreciable degradation of raloxifene (ca. 0.02%).15
|
Binder, 6% |
Raloxifene N-Oxide, %3 |
||||
|
Granule (Initial) |
Tablet |
||||
|
Initial |
1 mo. |
3 mo. |
6 mo. |
||
|
PEG-PVA |
0 |
0 |
0 |
0 |
0 |
|
PVP K30 LP (PeroXeal® packaging) |
0 |
0 |
0 |
0 |
0 |
|
PVP K30 (PeroXeal packaging) |
0 |
0 |
0 |
0 |
0 |
|
PVP K30 (PE in-liner) # 1 |
0 |
0 |
0.02 |
0.02 |
0.02 |
|
PVP K30 (PE in-liner) # 2 |
0 |
0 |
0.02 |
0.02 |
0.02 |
|
PVP K29/32 (Plasdone® K29/32) |
0 |
0 |
0.02 |
0.02 |
0.02 |
Table 5 Stability of raloxifene tablets stored at 40°C/75% RH2
Discussion
This study is aimed at examining the impact of peroxides on degradation of drug in formulations prepared by wet granulation and finds the appropriate excipients to mitigate the risks for degradation. API’s sensitivity toward residual peroxides on storage and during manufacturing could lead to detrimental effect on the stability and potency of drug products. Those residual peroxides typically originate during the manufacturing process and get carried over practically in many of the excipients used in formulation. These peroxides not only limited to binders and disintegrants such as povidone and crospovidone but to other ingredients such as fillers, lubricants and surfactants, can also cause oxidative degradation of sensitive drugs. Hartauer et al.,5 investigated the oxidation of raloxifene in tablets comprised of povidone as a binder and crospovidone as a disintegrant. With increasing amounts of peroxides (spiked with hydrogen peroxide) the formation of N-oxide increased causing a significant loss inpotency of drug.5 In our study, degradation of raloxifene in PEG-PVA matrix tablet was minimized due to lack of peroxides, as shown in Table 5. In a subsequent investigation, Yarkala et al.,16 studied the impact of a range of excipients used as binary mixtures of raloxifene in direct blend and wet granulation with povidone K30 and crospovidone (1:1), anhydrous lactose (5:1), and polysorbate 80 and magnesium stearate (10:1). The author observed that the N-oxide was formed from each of the binary mixtures of raloxifene on storage at 25°C, 40°C, 75°C, and 125°C but found that the increase was more significant with wet granulation due to intimate contact of drug and excipients, and was also dependent upon temperature.14 A further study suggests that the formation of N-oxide was not limited to formulation only but was also observed in the synthesis of raloxifene.17 Oxidative degradation of raloxifene may lead to adverse reactions.18 Thus, careful consideration should be given during the synthesis and formulation development of peroxide sensitive drugs like raloxifene and others to avoid the mechanical stress and risks for API’s degradation. Our data demonstrates that the PEG-PVA as a peroxide-free binder can withstand the robust processing conditions and can minimize the risk of oxidative degradation as evident from the data in Table 5.
Controlling the residual peroxides is therefore critical to improve long term stability and to maintain the quality of pharmaceutical dosage forms, and the research continues to find the optimal solutions. The possibilities include the use of antioxidants or smart packaging to alleviate the oxidative degradation.19,20 The data from this study demonstrates that PEG-PVA, lacking the inherent residual peroxides as shown in Table 4, curtails the oxidative degradation of raloxifene in the tablets. In contrast, the povidones (K29/32 or K30) with elevated levels of peroxides (>200 ppm), resulted in an appreciable degradation of drug in the tablets. Taken collectively, this study also demonstrates that PEG-PVA was exceptionally stable and did not show a peroxide increase under the various ambient stability conditions over a 5 years period.
PEG-PVA exhibits an excellent binding characteristic in fluid bed and high shear granulations. Our data demonstrate that the physical properties of granules and compressed tablets from PEG-PVA are comparable with the commonly used binders such as povidone K-30, copovidone and HPMC. The stability data reveal that the peroxide level does not increase at ambient and accelerated stability conditions. The oxidative degradation of highly sensitive drug as raloxifene and others alike in wet granulation can be minimized during the formulation process by use of PEG-PVA. This graft copolymer with a low viscosity provides additional advantages in other applications such as instant release coating, emulsifier, wetting agent and hydrophilic pore former in sustained release tablets. This study also provides an understanding on controlling the degradation of raloxifene and other drugs alike highly sensitive to peroxides by selecting the appropriate binders or excipients lacking residual peroxides. Thus, PEG-PVA with remarkable properties as binder and coating polymer, and free of peroxides, brings a new generation of excipient that could be widely applied to a range of wet granulation formulation development of highly sensitive drugs prone to oxidative degradation. With its approval in drug products and having listed in the FDA’s inactive ingredient database (IID), PEG-PVA opens doors to far more drugs sensitive to peroxides.
None.
Authors declare that there is no conflict of interest.

©2016 Fussnegger, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.