Journal of
eISSN: 2473-0831


Research Article Volume 12 Issue 1
1Chemistry Department, Haramaya Univeresity, Ethiopia
2Chemistry Department, RKSD (PG) College, India
3Chemistry Department, CCS Haryana Agricultural University, India
Correspondence: O.P. Yadav, Chemistry Department, CCS Haryana Agricultural University, Hisar-125004, India
Received: February 20, 2023 | Published: March 2, 2023
Citation: Etay H, Kumar A, Yadav OP. Kinetics of photocatalytic degradation of methylene blue dye in aqueous medium using ZnO nanoparticles under UV radiation. J Anal Pharm Res. 2023;12(1):32-37. DOI: 10.15406/japlr.2023.12.00421
Environmental pollution caused by untreated effluents from Paper, textile, printing, and several other industries is of serious concern. These industries generate a tremendous amount of wastewater contaminated with harmful chemicals. Methylene blue dye finds important application in pharmaceuticals to treat methemoglobinemia: a rare blood disorder. However, while present in drinking water or food material, methylene blue (MB) dye is a serious health hazard as it is carcinogenic and non-biodegradable that can cause a threat to human health and environmental safety. Hence, there is a need to develop an environmental friendly, and efficient technology for removing MB from wastewater. The usual physical and chemical methods employed for the removal of dyes are less efficient, costlier, and generate solid waste with adverse environmental impact. In the present work, Zinc oxide (ZnO) nanoparticles were synthesized by hydrolysis and oxidative method. As-synthesized ZnO powder was characterized using X-ray diffraction, and UV visible spectroscopic techniques. Photocatalytic degradation of methylene blue dye, catalyzed by as-synthesized ZnO nanoparticles, has been carried out in aqueous medium under UV radiation. The effects of operating parameters, such as amount of ZnO catalysts, pH, and initial concentration of dye on MB degradation, were investigated. The photo-catalytic degradation of MB was found to follow the pseudo-first-order kinetic model. Using ZnO optimum load 250 mgL-1, solution pH 10, the degradation of 20 mgL-1 MB was 85% at 3 hrs. Keeping in view its low cost, and high photocatalytic efficacy, the nanosize ZnO can be used for a large scale treatment of wastewater contaminated with methylene blue dye as pollutant.
Keywords: nanoparticles, methylene blue, kinetic, photocatalytic, degradation
Methylene blue, a thiazine dye, is used in pharmaceuticals as a medication to treat methemoglobinemia: a rare blood disorder, by converting ferric iron in hemoglobin to ferrous iron. Partricularly, it is used to treat methemoglobin levels that are greater than 30% or in which there are symptoms despite oxygen therapy. Methylene blue (MB) dye is also a highly consumed material in the dye, textile, and paper industries.1,2 Since MB is highly toxic, carcinogenic and non-biodegradable, its presence in wastewater can cause severe threat to human health as well as environmental safety. Ingestion of MB in human body through contaminated drinking water or food, can cause irritation of skin, mouth, and stomach; nausea, headache, vomiting, anemia & diarrhea.3 Therefore, the pre-treatment of wastewater contaminated with MB dye becomes necessary for maintaining the quality of water and its safer use.4,5 Wen et al.6 developed a potentiometric method for its use in pharmaceuticals for quantitative determination of MB in injections.
Untreated effluents from paper, dying, printing, and textile industries generate a tremendous amount of wastewater containing several health hazard chemicals. The usual methods employed for removing organic pollutants from wastewater include adsorption/biosorption7-9, coagulation/flocculation,10,11 phytoremediation,12,13 biodegradation,14-16 liquid-liquid extraction,17 electrocoagulation,18,19 ultrafilttration,20,21and nanofiltration.22,23 These methods are less efficient, costlier, and generate solid waste which requires secondary treatment.24
In the recent years, heterogeneous photo-catalytic degradation of recalcitrant organic compounds using semiconducting nanomaterial has received increased attention because of its high efficiency, cost-effectiveness, and environmental friendly process for treating wastewater.25-27 Zinc oxide (ZnO), due to its unique piezoelectric and ferromagnetic properties, has been used in the development of several devices of great practical use.28 Based on piezoelectric property of ZnO there are optoelectronic devices such as light emitting diodes (LEDs), solar cells,29 optical fibres etc. Ferromagnetic property of ZnO has been exploited in producing spintronics used in storing large data. Zinc oxide is also used in anti-UV radiation cosmetics, gas sensors, cancer detecting biosensors, and degradation of organic toxins.30 Zinc oxide due to its low cost, non-toxic nature, good stability, and high performance efficiency in photocatalysis have also drawn increasing attention for its use in the treatment of wastewater.31-33 Zinc oxide with band gap energy (3.37 eV), upon photexcitation in aqueous medium under UV radiation, generates hole (h+) with high oxidation potential. These reactive holes through several oxidative steps enable degradation of organic pollutants to generate simple non-toxic products such as CO2, H2O etc.
Acosta-Silva et al.34 used TiO2-decorated Santa Barbara Amorphous (SBA-15) mesoporous material for photocatalytic degradation of methylene blue (MB). Alamdari et al.35 green synthesized zinc oxide nanoparticles mediated by Sambucus ebulus leaf extract and used this material for photocatalytic degradation of methylene blue dye in the aqueous medium. The present work was carried out to investigate the effects of operational parameters such as catalyst load, solution pH, and dye initial concentration, on the kinetic of photocatalytic degradation of methylene blue under UV radiation.
Chemicals
Zinc chloride (ZnCl2; MW:136.32 g mol-1; hydrochloric acid (HCI, MW:36.5 g mol-1) were from Blulux Laboratories. Sodium hydroxide (NaOH, MW: 40 g mol-1, 98% pure), and Methylene blue (C16H18ClN3S, MW: 319.85 g mol-1) were procured from Breckland Scientific Supplies. The structural formula of methylene blue is shown in Figure 1.
Methods
Preparation of ZnO: Zinc oxide (ZnO) nanoparticles were prepared using hydrolysis and oxidation process. Typically, 0.M ZnCl2 solution taken in a glass beaker was mixed, dropwise, with 1M NaOH solution and the mixture was stirred until the pH of the solution reached to 12. White precipitates thus obtained were thoroughly washed with distilled water. The product was dried at 120 0C, and then calcined at 600°C for 3 hrs to get crystalline ZnO powder.36
X-ray diffraction study: The X-ray diffraction pattern of as-synthesized ZnO powder was recorded using an X-ray diffractometer (BRUKERD8 Advance XRD, AXS GMBH, Karisruhe, West Germany) equipped with Cu target for generating CuKα radiation (wavelength: 1.5406 A°). The applied accelerating voltage, and the current were 40 kV, and 30 mA, respectively. The instrument was operated under step scan type with step time, and 2θ degree as 1 sec, and 0.0200, respectively over 2θ range of 4° to 64°.
UV-visible absorption study: The UV-Visible diffuse absorption spectrum was recorded using a spectrophotometer (Perkin Elmer SP65). Typically, 0.2% aqueous dispersion (w/v) of ZnO was taken in a quartz tube, and the absorption spectra was recorded over 200-800 nm. The wavelength (nm) against the absorption peak maxima was recorded.
Photocatalytic degradation study: Photocatalytic degradation of methylene blue (MB) in its aqueous solution under UV radiation was carried out at ambient temperature. Typically, a known amount of the photocatalyst powder was thoroughly mixed with the MB dye solution of given concentration in a pyrex reactor tube. It was then put under UV lamp (Philips), predominantly emitting radiation at 365 nm. The UV lamp (with definite power 12 W; 230 Volts and 50 Hz frequency) was positioned parallel to the reactor tube at a distance of 6 cm. The desired pH of the reaction mixture was achieved by adding dropwise 0.1M NaOH or 0.1M HCl solution, and the reaction mixture was stirred at 3000 rpm to eliminate the external mass transfer effect. Before irradiating under UV light, the reaction mixture was magnetically stirred under dark for 15 minutes to establish adsorption/desorption equilibrium of methylene blue. Five ml each of reaction mixture was withdrawn after every 10 minutes, centrifuged at 4000 rpm for 20 minutes, and the concentration of methylene blue in the supernatant liquid was determined, spectrophotometrically, by recording absorbance at 665 nm.37
The percentage degradation of MB was calculated using equation 1.
Degradation (%) = (C0 - Ct)/C0)x100 (1)
Where, Co and Ct are the concentrations dye in solution at the initial stage, and at time t, respectively.
XRD analysis
The X-ray diffraction pattern of as-synthesized ZnO powder (Figure 2) depicting intense and narrow peaks indicates its crystalline nature. The diffraction peaks appearing at 2θ = 31.86, 34.54, 36.32, 47.62, 56.66 and 62.96 correspond to crystal planes (100), (002), (101), (102), (110) and (103) respectively, suggest the hexagonal wurtzite structure of the ZnO powder, and the same conforms with the JCPDS card, and other reports appeared in the literature.38 Non-appearance of additional diffraction peaks, due to any foreign material, ensured the purity of the prepared ZnO powder.
The average crystallite size of ZnO was obtained using Scherer formula39 represented by Eqn. 2.
D = [K λ/(βcos θ)] (2)
Where, D is the crystallite size in nm, K is the shape factor constant, taken as 0.94, β is the full width at half maximum (FWHM), in radians, for the most intense diffraction peak (at 2θ= 36.32) that corresponds to ZnO crystal plane (101), and λ is the wavelength of the X-ray (0.15406 nm), and θ represents the Bragg's angle. The values of β, and crystallite size of ZnO thus obtained were: 3.7x10-3 radians, and 41.2 nm, respectively.
3.2. UV/Visible Absorption Spectra
The UV/visible absorption spectrum of 0.2% ZnO aqueous dispersion (w/v) presented in Figure 3, exhibited the maximum absorption wavelength as 360 nm. The band gap energy of as-synthesized ZnO was obtained40 using Eqn. 3.
EeV = 1240/ λ (3)
Where, EeV represents band gap energy in electron volts, and λ is absorption wavelength in nm. The band gap energy thus calculated for synthesized ZnO nanoparticles was 3.44 eV.

Figure 3 UV-Visible diffuse absorption spectrum of Zinc oxide (ZnO) photocatalyst (absorption wavelength: 360 nm).
Kinetics of photocatalytic degradation
Batch experiments were performed for studying photocatalytic degradation of methylene blue (MB). To achieve maximum removal of MB from its aqueous solution, efforts were made to explore the required optimum catalyst load as well as the solution pH.
Effect of photocatalyst load: Plots of methylene blue percent degradation as a function of reaction time, using varying ZnO load, and MB initial concentration 20 mgL-1, under UV radiation are shown in Figure 4. It was observed that as the catalyst load was raised from 50 to 250 mgL-1, degradation of MB also increases till an optimum catalyst load (250 mg/L) was reached. But, upon further increasing the catalyst load to 300 mgL-1, the photocatalytic degradation of MB is decreased. The observed increase in MB degradation upon raising the catalyst load upto the optimum value 250 mgL-1 could be because of the increasing availability of active sites on the photocatalyst surface, and an increasing number of dye molecules adsorbed on the surface of the catalyst.41 It leads to an increase in the number of photo generated highly reactive oxidant holes (h+), hydroxyl, and superoxide radicals within the aqueous medium, which enable higher photocatalytic degradation of MB through a series of intermediate oxidative steps. However, when the concentration of ZnO catalyst was further increased above the limiting catalyst load (250 mgL-1), the degradation of MB was lowered due to (a) increasing interception or scattering of UV radiation by enhanced ZnO particle density, and (b) the decreasing catalyst’s specific surface area due to tendency of ZnO particles agglomeration at higher catalyst load. The percent removal of methylene blue dye from its 20 mg L-1 solution was as high as 80% at the end of 200 min.

Figure 4 Plots of percent degradation of 20mgL-1 methylene blue (MB) under UV radiation as a function of time at varying ZnO catalyst load, and a fixed pH 10.
Photocatalyst load dependent degradation kinetic: Photocatalyst load dependent kinetic of methylene blue (MB) degradation behavior was examined in the light of pseudo first order kinetic model that can be represented by equation (4):
ln(C0/Ct) = k.t (4)
Where, Co and Ct represent MB concentrations at initial stage (zero time) and at time t, respectively. Plots of ln(C0/Ct) versus time (t) for degradation of MB in its 20 mgL-1 aqueous solution at varying ZnO load are shown in Figure 5. The observed linearity in these plots proves the applicability of the pseudo first order kinetic model for the photocatalytic degradation of MB in aqueous medium over the studied range of catalyst load. The rate constant (k) for MB degradation at any catalyst (ZnO) load, was obtained from the slope of the corresponding plot. The half-life time (t1/2) of MB in solution was obtained using Eqn. 5,
Half-life time (t1/2) = 0.693/ k (5)
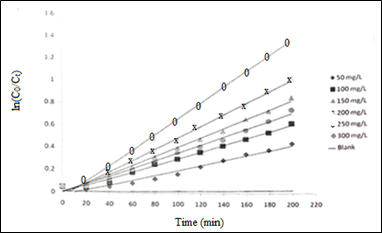
Figure 5 Plots of lnC0/Ct as a function of reaction time (min) at varying ZnO load (mgL-1); (MB initial concentration 20 mgL-1, and optimum pH 10.
The degradation rate constant k, and t1/2, at varying catalyst loads thus obtained, are recorded in Table 1, and also shown as plots of catalyst load versus k, and t1/2 in Figures 6A&6B, respectively. Photocatalytic degradation rate of MB gradually increases upon raising the catalyst (ZnO) amout till the optimum ZnO load 250 mgL-1 is reached where the maximum rate of dye degration 6.23x10-3 min-1, while at a higher catalyst load (300 mgL-1) it decreases to 5.97 x10-3 min-1. There is an obvious opposite trend in MB half-life time which decreases from 385.0 to 111.2 min till the optimum catalyst load is reached, and then it increases to 116.1 min at higher ZnO load.
|
[ZnO] (mgL-1) |
kx10+3(min-1) |
t1/2(min) |
|
50 |
1.80 |
385.0 |
|
100 |
2.94 |
235.7 |
|
150 |
3.94 |
175.9 |
|
200 |
4.89 |
141.7 |
|
250 |
6.23 |
111.2 |
|
300 |
5.97 |
116.1 |
Table 1 Pseudo first-order photocatalytic degradation rate constant (k) (min-1), and MB half-life time (t1/2) (min) in solution at varying photocatalyst (ZnO) load under UV radiation

Figure 6 (A) Plot of methylene blue photocatalytic degradation rate constant k(min-1) versus concentration of ZnO. (B) Plot of methylene blue half-life time t1/2 (min) versus concentration of ZnO. (MB dye initial concentration 20 mgL-1, optimum pH 10; under UV irradiation).
Effect of pH: Besides the need of optimizing the catalyst load, discussed above, for obtaining maximum degradation of organic pollutant such as MB, selection of optimum pH of the reaction medium is also important. It is because the pH affects both the nature and intensity of net electrical charge on the catalyst surface, and also the net charge on the substrate (MB) molecules. Both these factors affect the extent of adsorption of dye molecules at the photocatalyst (ZnO) guided by the level of ion-ion attraction or repulsion, which in turn affects the rate of photocatalytic molecular decomposition of the substrate (MB).42 Plots of percent photocatalytic degradation of MB as a function of reaction time, at varying pH of aqueous medium, is shown in Figure 7. Upon raising the solution pH from 7 to 10, photocatalytic degradation of MB increased from 48 % to 85 % at 3 hrs, but upon raising the pH at 11, MB degradation decreased to 70 %. With MB intial concentration 20 mgL-1, optimum catalyst load 250 mgL-1, and pH 10, maximum 85% degradation of MB was achieved under the UV radiation. The observed increasing MB degradation at higher pH (till the optimum pH) could be due to the increasing hydroxyl ions in aqueous medium which upon reacting with the photogenerated holes (h+) generate more hydroxyl radicals (.OH) needed for photocatalytic degradation of MB. The increase in MB degradation at higher pH may also be described in terms of variations of surface charge properties of the photocatalyst. Zinc oxide has point of zero charge (pzc) pH = 9.0 (pzc) pH = 9.0.43-44
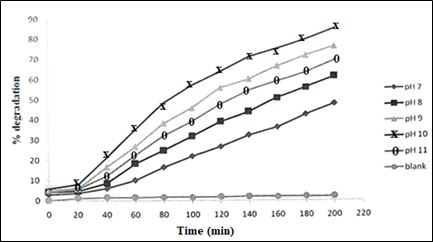
Figure 7 Plots of percent degradation of MB as a function of time at varying solution pH under UV radiation (MB initial concentration 20 mgL-1).
At a pH higher than pzc, the catalyst surface acquires negative charge and the reverse occurs at pH less than pzc. Since MB is a cationic dye, it obviously adsorbs more MB molecules at the negatively charged ZnO surface at higher pH. Further, since MB adsorption at the catalyst surface is a pre-requirement for photocatalytic reaction, the dye degradation would obviously be faster at higher pH. However, the decrease in MB degradation at still higher pH (=11) could be due to alkaline dissolution of ZnO.45,46
pH dependent degradation kinetic: The pH dependent MB photocatalytic degradation kinetic was studied following the pseudo first order kinetic model. Plots of ln(C0/C) as a function of time (min) at varying solution pH under UV radiation are shown in Figure 8. Photocatalytic degradation rate constant, k (min-1), and MB half-life, t1/2 (min) at varying pH of solution are given in Table 2, and the plot of MB photocatalytic degradation rate constant k(min-1) versus solution pH is shown in Figure 9A, and plot of methylene blue half-life time t1/2 (min) versus solution pH is given in Figure 9B. The maximum degradation rate of MB at the optimum pH=10 was found to be 7.02x10-3min-1 with MB half life time (t1/2) 98.7 min.
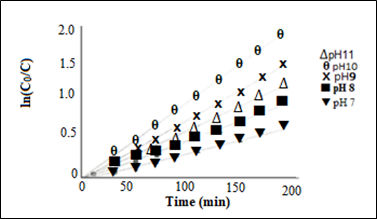
Figure 8 Plots of ln(C0/C) as a function of time (min) at varying solution pH under UV radiation [MB initial concentration (C0): 20 mgL-1; catalyst load : 250 mgL-1].
|
pH |
kx10+3 (min-1) |
t1/2 (min) |
|
7 |
3.00 |
231.0 |
|
8 |
3.93 |
176.3 |
|
9 |
5.43 |
127.2 |
|
10 |
7.02 |
98.7 |
|
11 |
6.67 |
103.9 |
Table 2 Pseudo first order photocatalytic degradation rate constant, k, and MB half-life, (t1/2) at varying pH of reaction mixture under UV radaiation
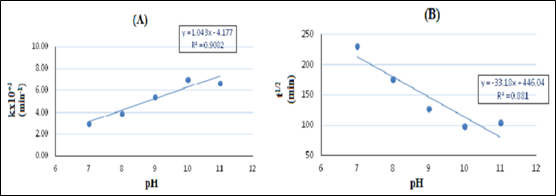
Figure 9 (A) Plot of methylene blue (MB) photocatalytic degradation rate constant k(min-1) versus solution pH. (B) Plot of methylene blue half-life time t1/2 (min) versus solution pH. [MB initial concentration (C0): 20 mgL-1, catalyst load: 250 mgL-1].
Effect of methylene blue concentration: To explore the effect of substrate (MB) initial concentration on its degradation, experiments were performed by varying initial concentrations MB from 20 to 100 mgL-1. The plots of MB percent degradation as a function of time (min) (optimum catalyst load: 250 mgL-1; pH: 10), using varying initial concentrations of MB, are shown in Figure 10. The percent degradation of MB was found to decrease with its increasing initial concentration. It could be because for the given catalyst load, with increasing concentration of MB, the availability of surface active site per dye molecule decreases. Besides it, at higher dye concentration, due to the absorption of some UV radiation by dye molecules in solution, the intensity of photons reaching at the catalyst surface diminishes leading to a decreased degradation rate of methylene blue.
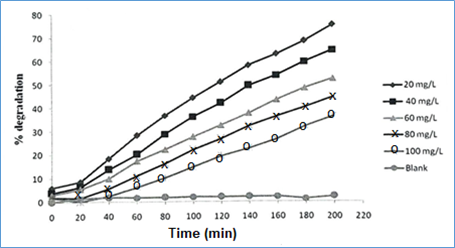
Figure 10 Percent degradation of MB dye under UV radiation as a function time using varying initial concentration (C0) of dye[optimum ZnO load: 250 mgL-1, and pH=10].
Dye concentration dependent degradation kinetic: The plots of In(Co/C) as a function of time (optimum catalyst load 250 mgL-1, and pH 10) using varying concentrations of MB, are shown in Figure 11. The observed linear plots of ln(Co/C) versus time, proves the applicability of pseudo first order kinetic model for dye degradation over its different studied intial concentrations. Values of MB photocatalytic degradation rate constant (k), and MB half-life time (t1/2) in solution, using varying initial concentration (C0) of MB, are given in Table 3. Plots of degradation rate constant (k), and MB half-life (t1/2), each as a function of MB intial concentration (C0), are shown in Figure 12A&12B, respectively. Whereas, the dye degradation rate decreases with increasing initial concentration (C0) of MB, the dye half-life time (t1/2) in solution has the reverse trend.

Figure 11 Plots of ln(C0/C) as a function of time (min) at different initial concentration (C0) of MB (optimum ZnO load: 250 mgL-1, and pH: 10).
|
MB initial concentration (C0)(mgL-1) |
Degradation rate constant kx103(min-1) |
MB half-life time t1/2 (min) |
|
20 |
5.29 |
131.0 |
|
40 |
4.14 |
167.4 |
|
60 |
3.15 |
220.0 |
|
80 |
2.17 |
319.3 |
|
100 |
1.32 |
525.0 |
Table 3 Photocatalytic degradation rate constants k (min-1), and half-life t1/2(min) of MB in solution at different initial concentrations (C0) of MB
Zinc oxide (ZnO) nanoparticles have been prepared and used as catalyst in studying kinetics of photocatalytic degradation of methylene blue (MB) dye in aqueous medium under UV radiation. The efficiency of dye degradation was found to increase with increasing catalyst load as well pH of solution upto a limiting value in each case. But the degradation of MB decreased with increasing dye initial concentration over its studied range. The photocatalytic degradation of MB well fits the pseudo-first-order kinetic model. Under the optimum conditions of catalyst load: 250 mgL-1, and pH: 10, the dye degradation rate under UV radiation was 5.29x10-3 min-1, and the maximum 85% degradation/ decolourization of MB could be achieved in three hours. Considering the low cost of as-synthesized zinc oxide (ZnO) catalyst, and its good photo-catalytic efficiency, it can be recommended for a large scale treatment of wastewater contaminated with methylene blue dye as pollutant.
None.
There is no conflicts of interest.

©2023 Etay, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.