Journal of
eISSN: 2473-0831


Review Article Volume 7 Issue 4
Correspondence: Lorean Madriz, Analysis and Catalysis and Electroanalysis laboratories, Sim, Tel 58 0212 906 3955
Received: February 26, 2018 | Published: July 6, 2018
Citation: Madrizm L, Vargas R. Key aspects of surface plasmon resonance spectroscopy for analytical chemistry applications. J Anal Pharm Res. 2018;7(4):412-415. DOI: 10.15406/japlr.2018.07.00259
The principles of the physical chemistry of surface plasmon resonance in metallic nanostructures have been briefly discussed. The application of this surface spectroscopy to the measurement of the concentration of chemical species is provided, after the exposure of the analytical chemistry detection strategies, the last one based on the consequences of the equation of the extinction spectrum derived from Gustav Mie's theory. Finally, the results of the detection of insecticides (dichlorvos and lindane) using nanoparticles of Au, Ag and Au-Ag alloy were reported as key examples to illustrate the advantages of synergistically combining the optical and surface properties of metals when forming alloys.
Keywords: plasmon, spectroscopy, nanoparticles, alloys, analytical chemistry
Metals have been of great interest in many areas as structural materials, but when they are converted into nanoparticles, they acquire different properties among which are their small size, large surface area and high adsorption capacity,1–3 which makes them promising for several analytical applications such as clinical, pharmaceutical, food and environmental. One of the most important aspects of metallic nanoparticles are their optical properties, which depend on their dimensions, shape, metal composition and surrounding environment.1–6 These properties are due to the principle of resonance of the surface plasmon (SPR) by which the electrons in the conduction layer of the material oscillate due to the frequency of electromagnetic radiation of the incident light.1–6 For this reason, in this mini review, the principles of surface plasmon resonance in metal nanostructures according to the Theory of Mie as well as the applications of this principle for the detection of analytes of interest will be considered, taking as an example the determination of dichlorvos and lindane by nanoparticles of Au, Ag and their alloys in order to illustrate the synergistic effect of using the latter.
Surface plasmon resonance (SPR) is used to measure binding events between molecules ranging from ions to viruses.1 Current technology provides molecular binding with information on kinetics, affinity and very sensitive concentration measurements, with key applications in surface-enhance spectroscopy and sensing.4 The SPR is related to the physical process that involves electromagnetic waves beyond the diffraction limit and up to the nanoscale. In general, the SPR phenomenon involves the formation of a polariton, which is a coherent oscillation that arises from the interaction of electromagnetic waves with the collective oscillations of free electrons on the surface of a metal.2,4 The surface plasmons are mainly limited by the size and shape of the metal structure that supports the plasmon and by the environment that defines the metal-dielectric interphase: the refractive index and the nature and concentration of the chemical species.2
On the basis of linear optics, the dielectric function of a material reflects key information about the interaction between its electrons and light, and it should be noted that the ability of a metal nanoparticle to support a surface plasmon depends on its dielectric function, which is an optical function in a complex variable where both the real and the imaginary part correlate with the refractive index of the material and its light absorption coefficient, the latter varies with the wavelength of excitation of the light. In general, materials with negative real and small positive imaginary dielectric constant, can experiment the SPR phenomenon.4
Figure 1A illustrates the local surface plasmon resonance mode (LSPR), in which the electromagnetic wave induces a force on the electrons of the metal conduction band and promotes its oscillations, nanostructures with dimensions smaller than the wavelength of the light support LSPR phenomenon. Figure 1B shows the propagation surface plasmon resonance mode (PSPR), in this case the metal structures have at least one dimension that approaches the excitation wavelength, and then the plasmons propagate for distances of the order of 10-100μm along the metal-dielectric interphase.4 Silver, gold, lead, copper, tin, aluminum and alloys make from these metals support the plasmon resonance phenomenon from the UV-Visible regions to the near IR of the electromagnetic spectrum.3,5
Following the ideas of Gustav Mie for the resolution of Maxwell's electromagnetic equations applied to very small conductive spheres, illuminated with z-polarized light at a wavelength greater than the characteristic length of the particles, it is possible to find the extinction (absorption+scattering) spectrum (E(λ)) of the particles immersed in a dielectric,4,5 see equation (1). For a detailed mathematical treatment of the problem it is suggested to read the mandatory review of Willets and Van Duyne and take a special attention to their supplementary material.4
(1)
where: a is the characteristic length (size) of the metal particles, λ is the wavelength of the light excitation, N is the density particles number, ɛout is the dielectric constant of the environment of the metal particles, ɛr(λ) is the real part of the dielectric function of the metal, ɛi(λ) is the imaginary part of the dielectric function of the metal and χ is the aspect factor of the nanoparticles (for a sphere: χ=2).4
For chemical analysis, a simple interpretation of equation 1 suggests that the spectrum of the system depends mainly on the refractive index of the medium, which is related to the concentration of the chemical species, as well as, the dielectric properties of the metal particle, which depends on their chemical nature, size and shape.2–6
According to this theory the wavelength maximum in the SPR spectrum (λmax) results sensitive to the dielectric constant of the phases, then changes in the local environment, as for example the adsorption of the chemical species on the metal surface, cause a shift in λmax that results proportional to the change in refractive index induced by the adsorbate, and the last should be correlate with the concentration of the analyte. The change in the wavelength maximum (Δλmax) results as follow:
(2)
where: m is the bulk refractive index response of the nanoparticle, Δn is the change in refractive index induced by the adsorbate, d is the effective adsorbate layer thickness, and ld is the electromagnetic field decay length.
Figure 2 summarizes these effects and illustrates the commonly used analytical chemistry detection strategies based on SPR phenomenon. Figure 2A indicates the SPR change by molecular adsorption on metal particle surface, Figure 2B illustrates the plasmon relation with the refractive index change of the bulk solution by the increment of chemical species concentration and Figure 2C shows that the synergic combination of optical and surface properties of different metals when they form alloys, can be used to changes the refractive index of the phases and enhances the molecular adsorption.
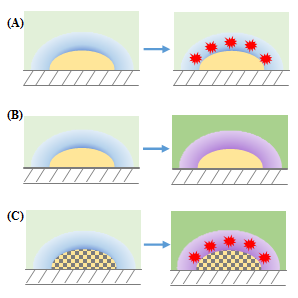
Figure 2 Analytical chemistry detection strategies: (A) molecular adsorption on metal particle surface, (B) refractive index change of the bulk solution by the increment of chemical species concentration and (C) synergic combination of optical and surface properties of metals when they form alloys: changes of the refractive index of the phases and molecular adsorption enhances.
Noble metals have been commonly used for fabrication of nanostructures which have led to advances in several areas of science; moreover, these nanostructures have been employed in construction of sensors based on different principles in order to be used in specific applications.1 One of the principles is SPR, which was described previously and allow avail the optical properties of materials for detect molecular interactions near the nanoparticle surface through shifts in the spectral peak.
Figure 3 shows the LSPR reflectance spectrum (A) and the wavelength maximum shift vs. concentration for dichlorvos measurements (B), both on Au, Ag and Au – Ag alloy nanoparticles electrocrystalized on screen printed graphite electrodes from DropSens® (for synthesis details, please see the works of Liu et al.32 and Tao et al.28). Instrumentation: for electrocrystallization a Bipotentiostat DropSens µSTAT200 and for spectrum measurements an Ocean Optics spectrometer s1024dw were used.
One way to increase the specificity of the sensors based on SPR, consist in modify it with biomolecules in order to detect interactions: biotin - streptavidin, antibody - antigen, nucleic acid hybridization, protein - carbohydrate, cytochrome - inhibitor, aptamer - protein, toxin - receptor,3,7–10 etc. Either sensors and biosensors, have applications in detection of drugs, anabolic steroid, opioids, stimulants and some peptide hormones in human organism,11,12 health,13–15 bacterias,16,17 viruses,18,19 allergens,20,21 pesticides,13,22,23 industrial safety,24,25 quality control,26–28 etc. The applications for sensing are very extends, please for more details see recent review of Boken et al.1 and the earlier review of Mayer and Hafner.3
As example of SPR detection, a particular case will be described, that is the use of screen printed graphite electrodes electrochemically modified with nanoparticles of Au, Ag and Au - Ag alloy in determination of dichlorvos and lindane. The first is an organophosphorus insecticide used mainly in storage areas and granaries, its use has been generalized due to its relatively low persistence (months) in the environment compared to other compounds of similar application, however, they have very acute toxicity and exposure to high levels can affects the nervous system;29 and the last is an organochlorine insecticide used to protect crops from pests, it is even used in lotions and shampoos to treat lice and scabies, its use is restricted around the world, due to its neurotoxicity and because it is carcinogenic and mutagenic.30 The importance of this studying lies in that the insecticides have been used to control pests and weeds, and their residues affect environment and human being, moreover, the traditional methods of detection such as gas and/or liquid chromatography are expensive, consume very time and required specialized operator.31Figure 3 shows the LSPR reflectance spectrum (A) and the wavelength maximum shift vs. concentration for dichlorvos measurements (B), both on Au, Ag and Au – Ag alloy nanoparticles electrocrystalized on screen printed graphite electrodes from DropSens® (for synthesis details, please see the works of Liu et al.32 and Tao et al.28). Instrumentation: for electrocrystallization a Bipotentiostat DropSens µSTAT200 and for spectrum measurements an Ocean Optics spectrometer s1024dw were used.
A briefly revision about the principle of plasmonic resonance spectroscopy was discussed and the strategies to use sensors based on this effect were raised, the new sensing opportunities arise from this effect were commented. The synergic effect of optical and surface properties of alloy metals was exemplify using the detection of insecticides as dichlorvos and lindane on Au–Ag alloy nanoparticles, which gave results into their range of interest for environmental and health risks in water solutions.
None.
The author declares that there is no conflict of interest.

©2018 Madrizm, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.