Journal of
eISSN: 2473-0831


Research Article Volume 3 Issue 2
Correspondence: Xin-Hui Jiang, Professor, School of Pharmacy, Chongqing Medical University, No.1 Yixueyuan Road, Chongqing, 400016, People?s Republic of China, Tel -68485098, Fax -68485098
Received: July 12, 2016 | Published: September 19, 2016
Citation: Cui T, Zhou Q, Zhang D, Jiang X (2016) Investigation the Distributions and Pharmacokinetics of Five Rhubarb Anthraquinones in Rabbits and Rats. J Anal Pharm Res 3(2): 00048. DOI: 10.15406/japlr.2016.03.00048
The main effective components of Rhubarb are anthraquinones (AQs), most of which are glycoside and others are free. The concentrations of AQs derivatives (rhein, aloe-emodin, emodin and chrysophanol) in plasma and homogenate were assayed with a high performance liquid chromatography (HPLC) method. The pharmacokinetic parameter and distributions of rhubarb AQs in rabbits or rats were obtained after given different formulas respectively. The elimination of AQs was fit to a two-compartment model in rats and rabbits. There were no significant differences in the main pharmacokinetic parameters between with rhein and AQs in rats. AQs were distributed in kidney, liver, blood, heart progressively. The AQs were mainly existed as rhein in vivo and excreted by kidney. To the formulas containing rhubarb, rhein could be used as a probe for pharmacokinetic study of Rhubarb AQs in vivo.
Keywords: rhubarb, anthraquinones, pharmacokinetics, distribution, chrysophanol, aloe-emodin, in vivo
Rhubarb (Dahuang in Chinese), a well-known chinese herbal medicine, has long been used in the world and is officially listed in Chinese Pharmacopoeia which containing three species, Rheum palmatum L., Rheum officinale Bail and Rheum tanguticum Maxim. ex Balf.1 It is also formally listed in European and Japanese Pharmacopoeia.2,3 This important herbal medicine has been discerned for centuries in traditional medicine for its multiple pharmacological actions include antibacterial, laxative, antispasmodic and hemostatic.4,5 The main effective components of Rhubarb are five anthraquinones, namely rhein, emodin, aloe-emodin, chrysophanol and physcion. Most of which are glycoside. More than 16 pure compounds which about 3-5% constituents of Rhubarb have been separated. These are the basis for the quality control of rhubarb and other plant drugs from Rheum, Cassia and Polygonum genera. The chemical structures of these AQs and internal standard (IS) are shown in Figure 1. We designed the experiment for tentative discussing the pharmacokinetic parameters and the excretion of Rhubarb AQs in vivo.
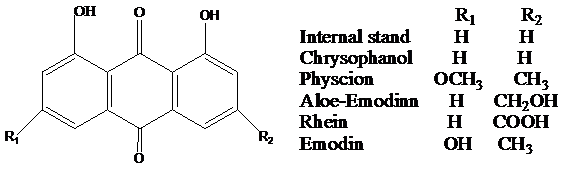
Figure 1 The chemical structures of rhein, aloe-emodin, emodin, internal standard, chrysophanol and physcion.
The methods commonly used for the separation and determination of all or some of the five major AQs in rhubarb are micellar electrokinetic chromatography (MEC),6,7 capillary zone electrophoresis8 and high performance liquid chromatography (HPLC).9–18
In our studies, we established a reliable and rapid method for simultaneous assay of five AQs in rhubarb on plasma and tissue homogenate and its related preparations using HPLC. Because of its sensitivity and speediness, HPLC is obtaining considerable attention in recent years for pharmaceutical and biomedical analysis. The results of our studies provided a reference for investigation of pharmacokinetics and distributions and of relations between pharmacokinetics, distributions and clinical efficacy of Rhubarb anthraquinones in human body.
Rhubarb (Dahuang in Chinese), a well-known chinese herbal medicine, has long been used in the world and is officially listed in Chinese Pharmacopoeia which containing three species, Rheum palmatum L., Rheum officinale Bail and Rheum tanguticum Maxim. ex Balf.1 It is also formally listed in European and Japanese Pharmacopoeia.2,3 This important herbal medicine has been discerned for centuries in traditional medicine for its multiple pharmacological actions include antibacterial, laxative, antispasmodic and hemostatic.4,5 The main effective components of Rhubarb are five anthraquinones, namely rhein, emodin, aloe-emodin, chrysophanol and physcion. Most of which are glycoside. More than 16 pure compounds which about 3-5% constituents of Rhubarb have been separated. These are the basis for the quality control of rhubarb and other plant drugs from Rheum, Cassia and Polygonum genera. The chemical structures of these AQs and internal standard (IS) are shown in Figure 1. We designed the experiment for tentative discussing the pharmacokinetic parameters and the excretion of Rhubarb AQs in vivo.
The methods commonly used for the separation and determination of all or some of the five major AQs in rhubarb are micellar electrokinetic chromatography (MEC),6,7 capillary zone electrophoresis8 and high performance liquid chromatography (HPLC).9–18
In our studies, we established a reliable and rapid method for simultaneous assay of five AQs in rhubarb on plasma and tissue homogenate and its related preparations using HPLC. Because of its sensitivity and speediness, HPLC is obtaining considerable attention in recent years for pharmaceutical and biomedical analysis. The results of our studies provided a reference for investigation of pharmacokinetics and distributions and of relations between pharmacokinetics, distributions and clinical efficacy of Rhubarb anthraquinones in human body.
Materials
Rhein, Aloe-emodin, Emodin, Chrysophanol and physcion were obtained from the national institutes for food and drug control, 1,8-dihydroxyan-thraquinone (internal standard, IS), acetonitrile, methanol, phosphoric acid, sulfuric acid, and ethyl ester were of analytical grade.
Subjects
6 healthy rabbits, males, weight 2.0kg - 3.0kg, and 30 male and 30 female SD rats, weight 150g-200g were purchased from School of Pharmacy, Chongqing Medical University, and R.P China. They were individually housed in stainless steel cages and acclimatized for 3 days prior to experiments which were kept on a 12 h light /dark cycle, allowed free access to water prior to oral gavage and without drinking during the experiment.
Method of oral gavage administration
WPY granule formula composed of Rhubarb, Ginseng, Betel nuts and Pharbitic was a novel drug which was used to treat acute pancreatitis. The particles contain Aloe-emodin 7.9824mg/g, rhein 12.2679mg/g, Emodin 7.3886mg/g, Chrysophanol 9.4768mg/g and Physcion 6.3278mg/g. The WPY dried powder was adjusted with water to equivalent as 0.763g original rhubarb herbs including rhein 4.50mg/mL. Preserved in refrigerator for rabbit and rats oral gavage.
The extract solution of Rhubarb was prepared as follow: Rhubarb was extracted twice with 70% ethanol, filtrated; the filtrate was concentrated to the relative density of 1.05 to 1.10. Which contained Aloe-emodin 4.0097mg/g, rhein 11.8765mg/g, Emodin 3.7676mg/g, Chrysophanol 3.3462mg/g and Physcion 2.3001mg/g? The liquid extract solution was adjusted to equivalent as 0.833g of native herbs including rhein 4.51mg/mL. Preserved in refrigerator for rabbits and rats oral gavage.
6 Healthy rabbits and 60 healthy SD rats were divided randomly into two groups, respectively. Blood was gotten and preserved in a tube which had added sodium heparin after rabbits had oral gavage of the extract solution of Rhubarb and the compounding formula solution of WPY respectively. Plasma and heart, liver and kidney tissues samples were preserved in refrigeration at -20°C till analysis within two weeks.
Preparation for plasma and tissues samples
Each biological sample was added 100mL internal standard solution and 1.0mL of 15% sulfuric acid and mixed. First, the samples were placed in 70±2°C water bath for 30min to hydrolysis conjunctive anthraquinones. Then, the samples were added 5.0mL ethyl ether to extract Rhubarb anthraquinones, after vigorous vortexing 3min, the samples were centrifuged at 10,000×g for 10 min at -4°C, the supernatant was drawn and dried with nitrogen at 40°C. Finally, the clear supernatant was reconstituted with 100mL mobile phase and 50mL supernatant was injected into the HPLC instruments.
Analytical method
The analytical method was established as follow: HPLC instrument with a UV detector (Shimadzu LC-2010) and Hypersil C18 column (250mm´4.6mm i.d. Dalian Elite Analytical Instruments Co., Ltd. China) were used for analysis. The mobile phase components were methanol- acetonitrile-water (10:50:40, v: v: v, adjusted to pH 2.8 with phosphoric acid), the detective wavelength was 225nm. The flow-rate was 1.0mL/min. Plasma and homogenate concentrations of anthraquinones were measured by using the established method. The ratios of peak areas of aloe-emodin, rhein, emodin and chrysophanol to I.S. were plotted versus the concentrations of aloe-emodin, rhein, emodin and chrysophanol.
Statistical and Pharmacokinetic analysis
The pharmacokinetic parameters in rabbits and rats, intergroup comparison were calculated by DAS version 2.0 which bought from the Center for Drug Clinical Evaluation of Anhui Medical University, China. Statistical analysis was performed with the two-sided t-test and paired samples test. As the plasma pharmacokinetic parameter of rhein after oral gavage displayed a typical bi-exponential decline, the plasma concentration-time data was fitted into the classical two-compartment first-order open model (C=Ae-αt+Be-βt). The plasma exposure (area under the plasma tetrandrine concentration-time curve (AUC0→24h), clearance (Cl0→24h), mean transit time (MTT0-24h) and terminal elimination half-life (t1/2) were calculated.
Anthraquinones existed form of before and after oral gavage administration
Most of the five anthraquinones are glycoside in Rhubarb, In order to determination the total anthraquinones we added 1.0mL of 15% sulfuric acid in each samples and placed it in 70±2°C water bath for 30min to hydrolysis conjunctive anthraquinones. The exits forms of anthraquinones in the extract solution of Rhubarb and WPY compounding formula solution before rabbits and rats oral gavage were shown in Figure 2 and that after oral were shown in Figure 2A-2F. From the chromatogram we can see that anthraquinones can be determined under the separation conditions and there were no interference.
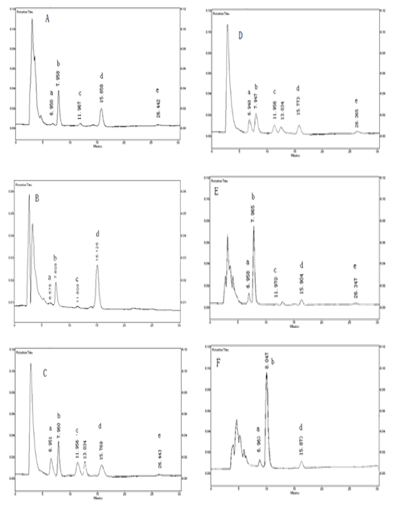
Figure 2 The chromatography of Rhubarb anthraquinones in rabbits and rats plasma were achieved on a Hypersil C18 column (10mm, 4.6mm×250mm i.d.). The mobile phase consisted of methanol, acetonitrile and water (10:50:40, v/v, pH2.8) at a flow rate of 1.0mL/min, and the detective wavelength was 225nm.
Figure 2A: A plasma sample taken from a rabbit after receiving an oral gavage of the extract solution of Rhubarb;
Figure 2B: A plasma sample taken from a rabbit after oral gavage of WPY,
Figure 2C: The extract solution of Rhubarb before oral administration;
Figure 2D: WPY before oral administration.
Figure 2E: A plasma sample taken from a rat after receiving an oral gavage of the extract solution of Rhubarb;
Figure 2F: A plasma sample taken from a rat after oral gavage of WPY.
A: aloe-emodin emodin, B: rhein, C: emodin, D: chrysophanol, E. Physcion
Pharmacokinetics after oral gavage administration
Plasma was gotten from ear vein after rabbits had oral gavage at 0, 0.25, 0.5, 1, 2, 3, 4, 6, 9, 12, 24 hours. The concentration of rhein in rabbits plasma vs time curves were shown in Figure 3. The pharmacokinetic parameters in rabbits after oral gavage of the extract solution of Rhubarb or compounding formula (WPY) were shown in Table 1. From Table 1 we can see that there were no significant differences (p<0.05) in the metabolism procedures of Rhubarb anthraquinones after rabbits had oral gavage different formulas. Plasma was obtained after rats had oral gavage of different formula at 5min, 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 12, 24hours. The concentration-time curves of rhein and total AQs in rat’s plasma were shown in Figure 4. We can know that rhein increased and others decreased in rabbits and rats after oral gavage of different formulas containing Rhubarb. The elimination of total AQs was fit to a two-compartment model in rats. The Pharmacokinetic parameters of rhein and its comparison result were listed in Table 2&3 and Figure 5. The comparison results of pharmacokinetic parameters of rhein in rats and in rabbits plasma were shown in Figure 5. From Figure 5 and Table 4 we can see that there were no significant differences in the main pharmacokinetic parameters in rats after oral gavage of different formulas containing Rhubarb. That is, the metabolism procedures of Rhubarb anthraquinones in different formulas can be described with rhein. From Figure 5 we can see that there were no significant differences between rats and rabbits after oral gavage of different formulas which containing Rhubarb anthraquinones from the metabolism procedures. Pharmacokinetic study on single-rhubarb and compound formula of anthraquinone derivatives showed that the metabolism in rabbits and rats are consistent with two-compartment model; the pharmacokinetic parameters calculated with rhein can represent the behalf of Rhubarb Anthraquinones metabolism in the biological process. The results of biological samples study can extrapolated that Rhubarb AQs in the metabolic process in vivo may be carried out as Figures 6&7 shown. Combination of oxidation and metabolism. Oxidation is the 3-methyl (Chrysophanol) have been oxidized to hydroxymethyl (aloe-emodin) and carboxyl (rhein).
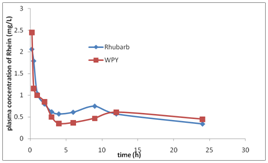
Figure 3 The curves of mean rhein concentration-time in rabbits plasma after oral gavage the extract solution of Rhubarb or WPY.
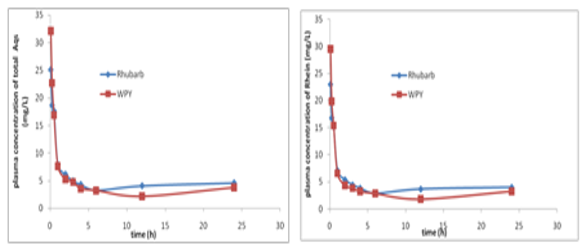
Figure 4 The curves of rhein or total AQs concentration-time in rats plasma after oral gavage of the extract solution of Rhubarb or WPY.
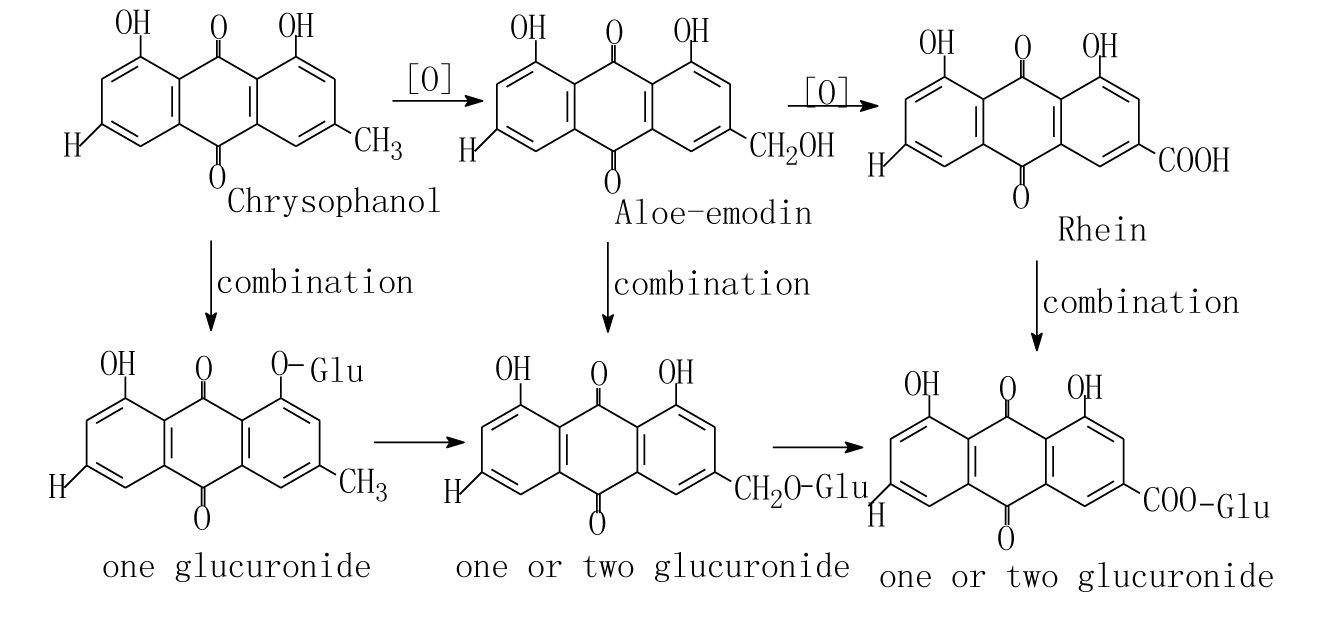
Figure 6 The metabolic pathway of chryophanol in vivo after rats oral gavage of different preparation containing Rhubarb.
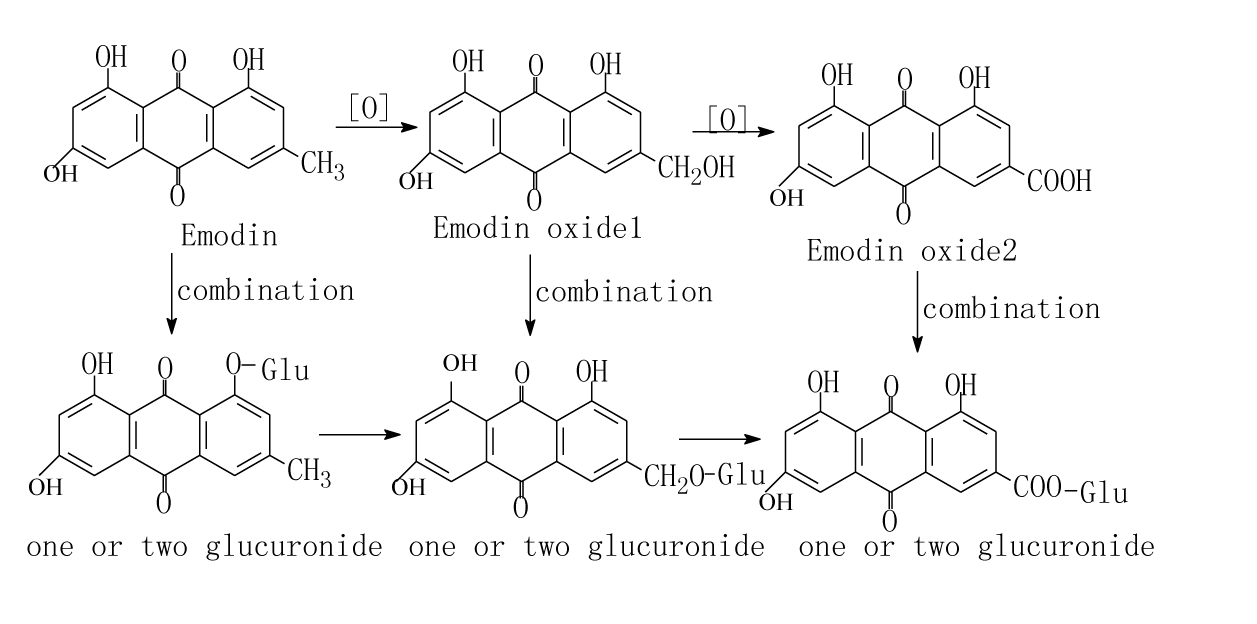
Figure 7 The metabolic pathway of emodin in vivo after rats oral gavage of different preparation containing Rhubarb.
|
Pharmacokinetic parameters |
Extract solution of rhubarb |
WPY |
|
compartment model |
two compartment |
two compartment |
|
Weighted Coefficient |
1 |
1 |
|
A (mg/L) |
4.1728±3.3881 |
6.4958±4.0837 |
|
α (1/ηρ) |
2.4617±1.0525 |
4.6488±4.2997 |
|
B (mg/L) |
0.7331±2.2870 |
0.6419±0.1647 |
|
β(1/ηρ) |
0.0247±0.0782 |
0.0250±0.0272 |
|
Ka (1/hr) |
6.5799±7.2936 |
13.4752±8.6081 |
|
V/F(c) (10ml/kg)/( mg/L) |
2.9922±4.0194 |
2.0427±2.4699 |
|
t1/2(α) (hr) |
0.2816±1.3778 |
0.1491±0.4757 |
|
T1/2(β) (hr) |
28.1055±16.2102 |
27.7543±22.8011 |
|
t1/2(ka) (hr) |
0.1053±0.2860 |
0.0514±0.0210 |
|
K21 (1/hr) |
0.5573±0.2609 |
0.6301±0.2036 |
|
K10 (1/hr) |
0.1089±0.0520 |
0.1843±0.1670 |
|
K12 (1/hr) |
1.8202±0.8233 |
3.8594±4.3104 |
|
AUC (mg/ml)*hr |
30.6755±4.5517 |
26.5689±8.6120 |
|
CL(S) (1ml/100g)/hr/( mg/L) |
0.3260±0.0437 |
0.3764±0.5739 |
|
T(peak) (hr) |
0.2800±3.1190 |
0.1275±0.0232 |
|
Cmax (mg/L) |
2.0453±1.2900 |
2.9512±1.7254 |
Table 1 The pharmacokinetic parameters in rabbits after oral gavage of the extract solution of Rhubarb and WPY respectively
|
Pharmacokinetic parameters |
Extract solution of rhubarb |
WPY |
|||
|
rhein |
Total of AQs |
rhein |
Total of AQs |
||
|
Compartment Model |
Two compartment |
||||
|
Weighted Coefficient |
1/C2 |
||||
|
A (mg/L) |
22.5419±20.8715 |
22.1741±10.3235 |
28.8972±13.7067 |
39.5559±6.6164 |
|
|
α (1/ηρ) |
1.4575±0.9118 |
1.3214±0.7375 |
1.9421±0.3740 |
2.3534±0.5111 |
|
|
B (mg/L) |
3.1455±0.4490 |
3.7652±1.5683 |
3.2091±1.2954 |
4.1697±0.8693 |
|
|
β(1/ηρ) |
-0.0119±0.0142 |
-0.0082±0.0407 |
0.0192±0.0336 |
0.0281±0.0071 |
|
|
Ka (1/hr) |
18.6264±39.2112 |
43.8505±1.9578 |
44.0966±89.1147 |
19.3712±38.2309 |
|
|
V/F(c) (1ml/100g)/ |
0.0418±0.0134 |
0.0396±0.0222 |
0.0324±0.0377 |
0.0257±0.0062 |
|
|
t1/2(a) (hr) |
0.4756±0.3074 |
0.5246±1.1632 |
0.3569±0.1153 |
0.2945±0.1140 |
|
|
t1/2(b) (hr) |
-58.1627±76.5560 |
-84.771±10.081 |
36.0917±29.4595 |
24.631±1221.592 |
|
|
t1/2(ka) (hr) |
0.0372±0.0635 |
0.0158±0.0182 |
0.0157±0.0131 |
0.0358±0.0079 |
|
|
K21(1/hr) |
0.1814±0.0671 |
0.1899±0.1395 |
0.2193±0.0586 |
0.2769±0.0170 |
|
|
K10 (1/hr) |
-0.0958±0.1280 |
-0.0569±0.4931 |
0.1701±0.3426 |
0.2391±0.1146 |
|
|
K<>sub>12 (1/hr) |
1.3599±0.9407 |
1.1801±0.9179 |
1.5720±0.0371 |
1.8654±0.5882 |
|
|
AUC ( mg/L)*hr |
26.0291±310.2810 |
4753.93±5752.36 |
181.248±115.889 |
3494.16±6416.72 |
|
|
CL(S) (1ml/100g)/hr/( mg/L) |
-0.0040±0.0053 |
-0.0023±0.0303 |
0.055±0.0256 |
0.0061±0.0039 |
|
|
T(peak) (hr) |
0.1583±0.1170 |
0.1698±0.1083 |
0.0746±0.0511 |
0.1364±0.0300 |
|
|
Cmax (mg/L) |
19.7035±1.2258 |
21.5728±2.1430 |
27.0116±13.6160 |
24.3986±5.3003 |
|
Table 2 Pharmacokinetic parameters with rhein or total AQs in rats after oral gavage of the extract solution of rhubarb and WPY respectively
|
No. |
AUC |
Tmax. |
Cmax. |
||||
|
Extract Solution of Rhubarb |
WPY |
Extract Solution of Rhubarb |
WPY |
Extract Solution of Rhubarb |
WPY |
||
|
x̄ |
33.16 |
34.4 |
0.18 |
0.11 |
2.78 |
3.34 |
|
|
SD |
4.55 |
25.29 |
0.15 |
0.02 |
1.29 |
1.73 |
|
|
2-tailed t-test |
t=17.86 |
t=3.33 |
t=3.07 |
t=3.61 |
t=5.29 |
T=4.74 |
|
|
t-test point value |
t0.05=4.303 t0.001=31.599 |
||||||
|
Paired t-test |
0.144 |
0.149 |
1.525 |
||||
|
Paired t-test P value |
0.895 |
0.294 |
0.405 |
||||
Table 3 The results of paired samples test of pharmacokinetic parameters in rabbits (rhein)
|
No. |
AUC |
Tmax. |
Cmax. |
||||
|
Extract Solution of Rhubarb |
WPY |
Extract Solution of Rhubarb |
WPY |
Extract Solution of Rhubarb |
WPY |
||
|
x̄ |
4753.93 |
3494.16 |
0.17 |
0.14 |
21.57 |
24.40 |
|
|
SD |
5752.36 |
26.07 |
0.11 |
0.03 |
2.14 |
0.12 |
|
|
2-tailed t-test |
t=2.02 |
t=-1.33 |
t=3.85 |
t=11.00 |
t=24.68 |
t=484.13 |
|
|
t-test point value |
t0.05=4.303 t0.001=31.599 |
||||||
|
Paired t-test |
1.784 |
1.025 |
3.085 |
||||
|
Paired t-test P value |
0.172 |
0.381 |
0.054 |
||||
Table 4 The results of paired samples test of pharmacokinetic parameters in rats (total AQs)
Tissue distribution study on rats after oral gavage
Tissue homogenates were obtained after rats had oral gavage different formula at 1, 2, 3, 4, 6, 12 hours. The concentrations of rhein in different tissues were obtained by HPLC and the data were shown in Table 5. From Table 5, we can see that total AQs and rhein were distributed in kidney, liver, blood and heart progressively. The anthraquinones were mainly existed as rhein in vivo and were excreted by the kidneys. The studies of distributions of Rhubarb anthraquinones were performed on 30 healthy rats after oral gavage of two formulas respectively. The results showed that Rhubarb anthraquinones in rats were distributed in kidney, liver, blood, heart progressively. It confirmed that the anthraquinones were mainly existed in rhein in vivo and excreted by kidney. The metabolic pathway of emodin may be described as Figure 7 shown. The metabolic processes can explain why the biological sample with a high concentration of rhein, and that of aloe-emodin and chrysophanol with low even do not detected.
|
Extract Solution of Rhubarb |
Samples |
Time (h)/Concentration (mg/kg) |
||||
|
1 |
2 |
4 |
6 |
12 |
||
|
Homogenates of heart |
1.42 |
5.59 |
4.25 |
1.36 |
4.03 |
|
|
4.79 |
6.38 |
1.81 |
1.55 |
0.28 |
||
|
20.64 |
9.76 |
0.49 |
0.56 |
0.35 |
||
|
Homogenates of liver |
2.49 |
2.67 |
1.96 |
1.83 |
2.78 |
|
|
4.56 |
5.56 |
3.86 |
0.9 |
2.95 |
||
|
0 |
10.1 |
0.88 |
1.09 |
1.64 |
||
|
Homogenates of kidney |
22.1 |
21.12 |
16.51 |
20.8 |
15.58 |
|
|
28.81 |
12.77 |
7.4 |
1.07 |
1.79 |
||
|
11.73 |
15.33 |
2.13 |
1.14 |
0.14 |
||
|
WPY |
Homogenates of heart |
3.46 |
2.55 |
2.68 |
4.38 |
0.62 |
|
6.36 |
2.53 |
3.88 |
0.83 |
0.39 |
||
|
3.47 |
8.02 |
0.69 |
0.17 |
0.93 |
||
|
Homogenates of liver |
7.07 |
6.38 |
2.42 |
5.99 |
3.45 |
|
|
2.58 |
0.93 |
4.04 |
2.63 |
4.18 |
||
|
1.07 |
8.02 |
1.19 |
0.86 |
1.16 |
||
|
Homogenates of kidney |
35.71 |
15.06 |
13.23 |
14.77 |
11.84 |
|
|
7.36 |
3.91 |
14.41 |
2.6 |
2.71 |
||
|
14.67 |
11.38 |
2.24 |
0.83 |
1.42 |
||
Table 5 The data of rhein concentration in 30 rats after oral gavage of the extract solution of Rhubarb and WPY respectively
In view of the complexity of Chinese herbal compound, a comprehensive picture of what all of its chemical constituents of the absorption, distribution, metabolism and excretion of the law is very difficult. To clarify the effectiveness of components can be detected and their dynamic changes in vivo, the efficacy of Chinese medicine composed of clear material basis, Compatibility of the law reform and clinical forms are useful medication.
The results of our studies could provide references for investigation of pharmacokinetics and distributions and of relations between pharmacokinetics, distributions and clinical efficacy of Rhubarb anthraquinones in human body, and provide a new thinking method for exploit and application of the traditional chinese medicine preparations. And to the preparations containing Rhubarb, rhein could be used as a probe for pharmacokinetic study of anthraquinones in vivo (Figure 8).
Thanks are due to Professor Jia Y.R. for Rhubarb of Cangdu Rheum PalmatumL and WPY provided.
The authors declare no conflict of interest.
None.

©2016 Cui, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.