Journal of
eISSN: 2473-0831


Research Article Volume 12 Issue 2
1Department of Chemistry, School of Physical and Mineral Sciences, Faculty of Science and Agriculture, University of Limpopo (Turfloop Campus), Private Bag X1106, Sovenga 0727, South Africa
2Department of Physics, Materials Modelling Centre, School of Physical and Mineral Sciences, Faculty of Science and Agriculture, University of Limpopo (Turfloop Campus), Private Bag X1106, Sovenga 0727, South Africa
Correspondence: Vusimuzi Ludwig Mulaudzi, School of Physical and Mineral Sciences, Faculty of Science and Agriculture, University of Limpopo (Turfloop Campus), Polokwane, Private Bag X1106, Sovenga 0727, South Africa
Received: April 15, 2023 | Published: May 2, 2023
Citation: Pesha T, Mulaudzi VL, Mkhonto PP, et al. Inhibition of zinc corrosion by glycerol stearate in 1.0M hydrochloric acid medium with experimental, theoretical, and electrochemical techniques. J Anal Pharm Res. 2023;12(2):60-66. DOI: 10.15406/japlr.2023.12.00424
Glycerol Stearate (GS) was investigated as a corrosion inhibitor via weight loss on zinc (Zn) metal inside 1.0M hydrochloric acids (HCl). Different electrochemical techniques such as potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS) and characterization methods were used in the study. Weight loss measurements and inhibition efficiency (IE) were used to calculate the amount of weight loss and to scrutinize the effect of inhibition concentration on the metal in HCl, and it demonstrated that weight loss decreased as the inhibition concentration increased, and percentage inhibition efficiency increased with increasing inhibition concentration. The Corrosion rate () was calculated, and it was observed that it decreased with the increasing inhibition concentration but increased with an increase in temperature. The compound of inhibitor effectively prevented corrosion by becoming adsorbed to the metal surface and was confirmed by Fourier transform infrared spectroscopy (FTIR). Free Gibbs energy (FGE) demonstrated a spontaneous corrosion process at the metal surface of zinc and the scanning electron microscope (SEM) was used to investigate the surface morphology of the protective layer and confirmed that the adsorption of glycerol stearate was via physisorption adsorption. The adsorption of glycerol stearate on the metal surface was found to follow the Langmuir adsorption isotherm model.
Keywords: zinc metal, glycerol stearate, corrosion inhibition, weight loss, corrosion rate, adsorption, inhibitor concentration, inhibition efficiency
Corrosion is the natural process that occurs when native metals are transformed into undesirable substances when they react with substances like water or air.1 This process undergoes a redox reaction whereby metals act as the reducing agent, losing electrons, and the oxygen (in water and air) act as the oxidizing agent, receiving electrons, causing the reaction to be spontaneous and electrochemically favoured. The damage to the metals caused by this reaction begins from the tiny part of the metal exposed to a corrosive environment which then leads to rusting.2 When the metal is subjected to the rusting, hydroxyl ions (OH) occur when the electrons on the oxygen, and the hydrous iron oxide Fe(OH)3 is formed through the reaction of hydroxyl ions and Fe3⁺: Fe3+ + 3OH- → Fe (OH)3 (Overall equation).
Corrosion has been identified as a major problem in olefin applications due to the loss of materials such as pipes, especially in oilfields. Oil and gas-producing industries are negatively affected by corrosion worldwide every year, which results in a great loss of costs and a negative impact on the economy.3 The economic loss of corrosion worldwide is estimated to be greater than $2.5 trillion, while in South Africa it is R130 billion.4 This was conducted by the University of Witwatersrand and the Corrosion Institute of Southern Africa. Most companies transport the products through pipelines and tanks, which get into contact with water and interact with air. After the reaction of oxygen and water, the rust is generated which then flows with the compressed air inside the steel pipes, attacking them from the small portion to the entire metal. The use of inhibitors has been identified as the method for preventing corrosion.
Corrosion inhibitors are substances that prevent or decrease the rate of corrosion on metals.5 This is done by coating, plating, cathodic protection, and anodic protection of the surface of the metal or by immersing the metal inside the inhibitor solution. In addition, sodium hydroxide (NaOH) and sodium carbonate (Na2CO3) are the most used inhibitors; since they increase pH near metals which reduces the transportation of oxygen to the metal surface. Other organic compounds such as heterocyclic compounds are effective and form a hydrophobic film on the metal surface. In this study, the use of the glycerol stearate in Figure 1 is reckoned as a part of the green chemistry inhibition method. The concentrations of the inhibitor decrease the rate of corrosion, especially the higher concentrations than the lower ones. The prevention occurs by immersing the metal inside the inhibition solution.6 The metal absorbs the inhibitor on its surface, forming a protective film and preventing oxygen or water to attack. Furthermore, temperature has been identified as one of the factors affecting the rate of corrosion.
Glycerol stearate was purchased from Prestige laboratory supplies, South Africa. Zinc metal sheets were prepared and purchased from the University of Northwest, South Africa, and 32% Hydrochloric acid (HCl) was purchased at Rochelle Chemicals, South Africa.
Gravimetric method
Three zinc metals with the specimen of 3 cm x 4 cm were weighed and immersed inside 100 ml beakers containing a blank solution of 1.0 M prepared from 32% Hydrochloric acid. The metals were again completely immersed inside 100 ml beakers containing different glycerol stearate concentrations, 10 x 10-5 M, 30 x10-5 M and 50 x 10-5 M inside 1.0 M Hydrochloric acid and placed inside three thermostats at temperatures of 318 K, 328 K and 338 K, constituting three sets of experiments. After the time has elapsed, the metals were removed from the thermostats and washed with distilled water, then rinsed with acetone. They were dried for 5 to 10 minutes then re-weighed, and the final masses were recorded. The experimental time for metal immersion in the absence and presence of an inhibitor was 3 hours.
The corrosion rate (ρ in g.cm−2.h−1), percentage inhibition efficiency (%I), and surface coverage (θ) were calculated from the weight loss using the equations below.7
(1)
(2)
(3)
Where denoted zinc average weight loss, S denoted total surface area of the zinc specimen (cm2), and t denoted immersion time (h), while and were corrosion rates with and without inhibitors respectively.
Characterization technique
The inhibitor was confirmed by the Spectrum II FTIR spectrometer (PerkinElmer). The spectrum was within 500 and 4000 cm-1 at a resolution of 4 cm-1. Scanning electron microscopy (SEM) was used to perform morphological analysis on the samples. SEM was used on a TESCAN Vega TC using TESCAN software, together with energy dispersive X-ray spectroscopy (EDXS) to determine the elemental composition of the samples (at 20 kV). The samples were gold-coated to enhance imaging by forming a conductive layer on the analytes' surfaces, which prevented charging and elemental composition interference.
Adsorption studies
During the corrosion testing in the presence of the inhibitor compound, an adsorptive film was formed on a zinc surface, and an oxide layer was further characterized using the FTIR technique, which was essential in studying the protective layer formed on the metal surface. This technique allowed for the analysis of both liquid and solid samples. In the FTIR technique, the infrared absorption spectrum was measured, and another advantage was that the technique is fast in processing data. Furthermore, the functional groups of the chemical inhibitor molecule were investigated using FTIR qualitative analysis.
Electrochemical measurement
Electrochemical parameters were obtained with the aid of the Bio-Logic SP150 potentiostat working station, which consisted of a three-electrode cell, namely, saturated calomel electrode (SCE), reference electrode (RE), platinum counter electrode (CE) and Zn metal (1cm2) working electrode (WE) without and with corrosion inhibitor concentrations. Potentiodynamic polarization studies were conducted between -0.250 and +0.250 mV scanning range at a 1.0 mV/s constant sweep rate on the open circuit potential (OCP). Both anodic and cathodic polarization graphs were achieved when stabilization was attained after the working electrode was immersed for 30 min in the test solution. Electrochemical impedance (EIS) measurements were conducted by employing a 100 kHz to 10 Hz frequency range with a 10 mV peak-to-peak voltage, using an alternating current (AC) signal at corrosion potential.
Potentiodynamic polarization
To obtain the relevant electrochemical parameters such as the anodic Tafel slope, and cathodic Tafel slope, , the potentiodynamic polarization method was used. According to Equation 4, the current densities measured were used to calculate the percentage inhibition efficiency of the chemical corrosion inhibitor compound.8
(4)
corrosion current density values were denoted by and denoting the corrosion current density in the absence and in the presence of an inhibitor respectively.
Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy was utilized for studying charge transfer resistance occurring during the corrosion of zinc metal in a corrosive environment. This technique was used to evaluate electrochemical measurements including solution resistance (Rs), charge transfer resistance ( ) with the inhibitor and charge transfer resistance ( ) without the inhibitor, double layer capacitance ( ), the constant phase element (CPE), and exponents, which were further investigated by employing EIS. Furthermore, Equation 5, and were used to calculate the inhibition efficiency:8
(5)
Weight loss measurements
Effect of inhibitor concentration and temperature on corrosion rate
The gravimetric experiments of glycerol stearate (GS) inhibitor were presented by the percentage inhibition efficiencies (%IE) against inhibitor concentration plots at 318, 328 and 338 K as given in Figure 2A. The %IE was observed to increase as the inhibitor concentration increased from 10 x 10-5 - 50 x 10-5 M, for GS. In addition, it was observed that the %IE decreased with the increase in temperature. The effect of temperature was shown by the %IE values for 10 x 10-5 M at 318 K, 67.16% while at 328 and 338 K, 68.65% and 73.13% were obtained respectively. The density of the corrosion rate increased severely as the temperature increased. As shown from studies, the rate of metal dissolution was hindered by increasing the inhibitor concentration. The corrosion rate was found to be 15.56, 19.44 and 31.11 × 10-3 g.cm-2.h-1 in the uninhibited solution at 318, 328 and 338 K, respectively. However, according to the observations in Table 1 it was observed that upon the introduction of the inhibitor in the solution, the rate of corrosion decreased. A similar behaviour was observed by Hong et al.9 using fungicides and 4-amino-antipyrine on the corrosion of copper in NaCl solution, respectively. It was found that the metal dissolution decreased optimum to a value of 10.0× 10-3g. cm-2. h-1 in GS for 50 x 10-5 M inhibitor concentration at 318 K. Furthermore, metal weight loss decreased with a decrease in the rate of corrosion due to the inhibitor adsorption on Zn metal surface.
|
Inhibitor |
Temperature(K) |
Concentration(x 10-5 M) |
Weight loss (g) |
Corrosion rate (x 10-3 g. cm-2. hr-1) |
Inhibition efficiency (IE) |
Surface coverage ( ϴ) |
C/ϴ x 10-5 |
|
GS |
318 |
0 |
0.28 |
15.56 |
_ |
_ |
_ |
|
10 |
0.22 |
12.22 |
67.16 |
0.6716 |
14.8893 |
||
|
30 |
0.21 |
11.67 |
68.65 |
0.6865 |
43.6968 |
||
|
50 |
0.18 |
10.0 |
73.13 |
0.7313 |
68.3688 |
||
|
328 |
0 |
0.35 |
19.44 |
_ |
_ |
_ |
|
|
10 |
0.32 |
17.78 |
56.17 |
0.5617 |
17.8034 |
||
|
30 |
0.30 |
16.67 |
58.91 |
0.5891 |
50.9263 |
||
|
50 |
0.29 |
16.11 |
60.28 |
0.6028 |
82.9486 |
||
|
338 |
0 |
0.56 |
31.11 |
_ |
_ |
_ |
|
|
10 |
0.45 |
25.00 |
39.19 |
0.3919 |
25.5183 |
||
|
30 |
0.44 |
24.44 |
40.54 |
0.4054 |
74.0029 |
||
|
50 |
0.43 |
23.89 |
41.89 |
0.4189 |
119.3593 |
Table 1 Corrosion rate (ρ), the efficiency of inhibition, (%IE) and surface coverage (θ) of GS at 318, 328 and 338 K for zinc metal
The mechanism type followed during the process of adsorption at the inhibitor/metal interface has been investigated in a significant way by fitting various adsorption isotherms with the best regression line R2 value. Figure 2B depicted a Langmuir adsorption isotherm plot with R2 values ranging from 0, 9996 to 0, 9999. The Langmuir plots were enhanced by R2 values close to unity. Table 2 showed the adsorption equilibrium constant and standard free energy of adsorption values. The number of inhibitor layers that had been adsorbed on the metal surface was calculated by using the slopes of the regression lines. The standard free energy of adsorption.10 offered insight into the spontaneity of the inhibitory process as well as the stability of adsorption. According to the research that has been done.11 A spontaneous process was characterized by having values of free energy of adsorption that are on the negative sign. In addition, a physisorption adsorption mechanism is indicated when the value of the free energy of adsorption is less than or equal to -20 kJ. mol-1, whereas a chemisorption adsorption mechanism is indicated when the value is greater than or equal to -40 kJ. mol-1 in the negative direction.12 GS supplied a physisorption process, which can be found displayed in Table 2, for Gibbs free energy values that were lower than -20 kJ. mol-1.
|
Inhibitor |
Temperature (K) |
Adsorption equilibrium constant(x105 L.mol-1) |
Coefficient of determination |
Free Gibbs energy(kJ.mol-1) |
|
GS |
318 |
0.4528 |
0.9989 |
-8.52 |
|
328 |
0.5881 |
0.9999 |
-8.79 |
|
|
338 |
0.3877 |
0.9996 |
-9.06 |
Table 2 Adsorption parameters for glycerol stearate on zinc
Thermodynamic and activation parameters
Metal dissolution increased with the increase in temperature, and as a result, there was a lower activation barrier.13 With the help of the Arrhenius equation and plot, the effect of temperature on the adsorption of GS onto the Zn surface was evaluated. The log ρ against the 1/T plot was shown in Figure 2C. The plot assisted in calculating the values of the activation energy for the corrosion process. Table 3 showed records of the calculated parameters of activation energy with the aid of Equation 6. The activation energy value in the uninhibited solution was less than those obtained in the inhibited solution. Higher activation energy values in the inhibited solution advocated a prolonged rate of corrosion due to the formation of the GS/Zn complex.14 The entropy and enthalpy of activation can be used to investigate the inhibition efficiency of GS on the Zn metal surface. Scientists have found that higher negative entropy values represented less surface destruction on metal, while higher positive entropy values represented greater disorder in the system.15
(6)
|
Inhibitor |
Concentration (x10-5M) |
Activation energy (kJ.mol-1) |
enthalpy of activation (kJ.mol-1) |
Entropy (JK-1.mol-1) |
|
GS |
0 |
30.85 |
28.13 |
-197.30 |
|
10 |
31.98 |
29.26 |
-197.20 |
|
|
30 |
33.03 |
30.30 |
-197.05 |
|
|
50 |
38.94 |
36.21 |
-196.14 |
Table 3 Presented are activation energy (Ea), entropy (∆So) and enthalpy of activation (∆Hoa) values for zinc metal
Enthalpy values can represent either endothermic or exothermic reactions, depending on the sign of the value. Adsorption can be either physically or chemically involved in exothermic processes.16,17 The plot of the transition was shown in Figure 2 (D) with the aid of Equation 7:
(7)
Potentiodynamic polarization (PDP)
The polarization parameters can be determined with the help of tafel plots. These include the corrosion potential ( ), the corrosion current density ( ), the anodic tafel slope ( ), and the cathodic tafel slope ( ). Corrosion current densities were calculated by extrapolating tafel segments from anodic and cathodic curves. Coefficients of inhibition were found to be proportional to densities of corrosion currents (Equation 4).18 In Figure 3 shown was the tafel plot for zinc metal in 1.0M HCl at varying concentrations of the GS inhibitor compound, both in its uninhibited form and its inhibited form. The addition of glycerol stearate corrosion inhibitor was found to decrease values. Inhibitor adsorption onto the zinc metal surface was observed, providing support for the adsorption mechanism.18 Furthermore, the calculated difference between the blank (1. 0 M HCl) and the inhibitor solutions was less than 85 mV, which indicated a mixed-type mechanism of inhibition with the cathodic mechanism dominating as observed from the tafel slopes at each inhibitor concentration (Table 4).8
|
Inhibitor |
Concentration x 10-5 (M) |
-Ecorr (mV) |
Icorr (mA.cm-2) |
ba (mV) |
bc (mV) |
%IEPDP |
|
Blank |
|
445.25 |
0.38 |
70.3 |
97.5 |
- |
|
GS |
10 |
460.79 |
0.28 |
36.1 |
40.3 |
26.32 |
|
30 |
435.47 |
0.19 |
18.5 |
25.3 |
50.00 |
|
|
50 |
499.87 |
0.11 |
69.4 |
91.5 |
71.05 |
Table 4 Polarization measurements such as Ecorr, Icorr, ba and bc using different inhibitor concentrations
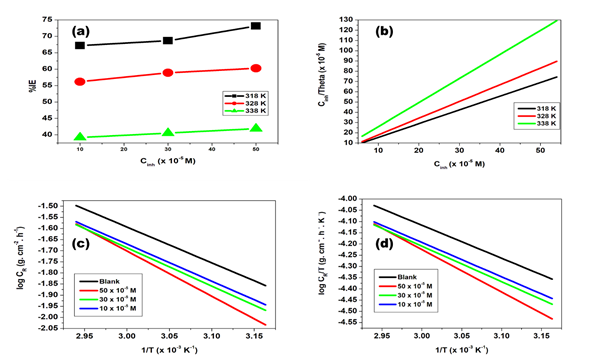
Figure 2 Efficiency (%IE) versus GS concentration (M) plot for (a) GS; and Langmuir isotherm (b) GS inhibitor on a zinc sheet at 318 K, 328 K and 338 K. Arrhenius graphs for zinc metal in 1. 0 M HCl with and without GS (c) Transition state graphs at differing GS (d).
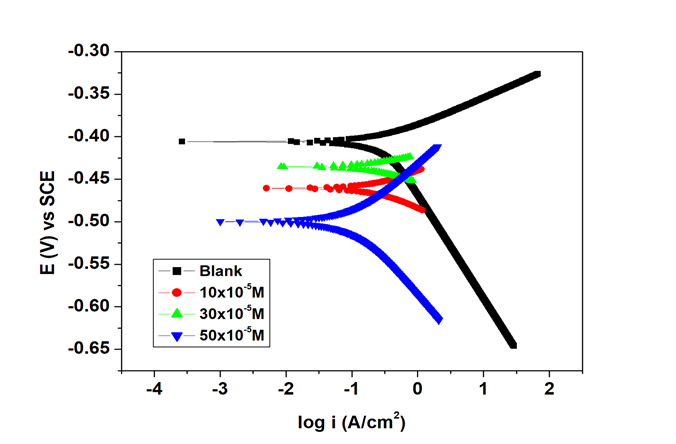
Figure 3 Potentiodynamic polarization plot for zinc in 1. 0 M HCl in the uninhibited and inhibited solutions of GS different concentrations.
Electrochemical impedance
Further study of corrosion behaviour zinc metal in acidic medium in the uninhibited and inhibited solution of GS at different concentrations was conducted. In Figure 4 & 5 shown was a representation of Nyquist plot and its corresponding bode plot for zinc metal in the absence and presence of GS inhibitor compound. Bode plots revealed some information with regards to electrochemical behaviour of both the uninhibited and inhibited systems, it was revealed that at higher phase angle there was a frequency shift to higher frequency in the presence of glycerol stearate concentrations. It was studied that the imperfection of the semicircles in the impedance spectra of zinc was due to the roughness and inhomogeneity on the metal surface.18 From Table 5 shown was the impedance data for zinc metal obtained. Table 5 showed that the charge transfer resistance increased as the inhibitor concentration increased. The highest efficiency of 89.50% was obtained at 50 x 10-5 M. Moreover, to define the impedance nature, an equivalent electric circuit made up of the solution resistor (Rs), charge transfer resistance ( ) and a double layer capacitance ( ) in Figure 6 was utilized. Furthermore, Figure 5 showed bode plots with phase angle of approximately 70 oC at the highest inhibitor concentration. Moreover, the phase angle was observed to increase as the inhibitor concentration increased and this was complimented by the frequency plot in Figure 5.
|
Inhibitor |
Concentration x 10-5 (M) |
Solution resistance (Ω) |
Charge transfer resistance (Ω) |
Double layer capacitance(x10-6 F) |
Inhibition efficiency |
|
Blank |
|
2.348 |
6.429 |
0.418 |
- |
|
GS
|
10 |
2.344 |
11.54 |
0.252 |
44.29 |
|
30 |
3.109 |
16.94 |
0.419 |
62.05 |
|
|
50 |
2.121 |
61.24 |
0.006 |
89.50 |
Table 5 Electrochemical impedance parameters
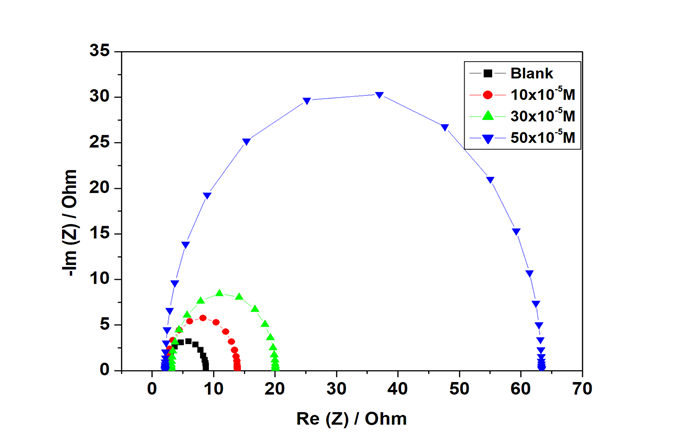
Figure 4 Nyquist plot for zinc in 1. 0 M HCl in the uninhibited and inhibited solution with different GS concentrations.
Adsorption studies
In the FTIR spectrum (Figure 7) of the adsorption film, a disappearance of the following bands was observed, such as hydrogen bonds (3307 cm-1 and 3241 cm-1) formed by hydroxyl group, CH2 stretch of alkyl carbon chains (2914 cm-1 and 2849 cm-1), another visible peak occurring at 1730 cm-1 accredited to the C=O stretching mode. This behaviour owed to the successful interaction between glycerol stearate and the zinc metal surface.
Morphological studies
Figure 8 displayed the pristine zinc metal with about 95.1% composition as shown by the EDXS spectrum, in addition, the micrograph showed smooth surface abrasions caused by the emery papers. Figure 9 displayed a micrograph with a tempered surface due to the presence of the Cl- (present in 6.8% composition shown on the EDXS) ion from the hydrochloric solution which facilitated the zinc metal dissolution. Finally, Figure 10 displayed SEM micrograph with the formation of agglomerations due to the presence of the glycerol stearate with the O atom (15.8% composition) which reduced the Cl- ion to 0.7% composition as compared to Figure 9 thus a successful inhibition of the metal dissolution.
Glycerol Stearate was found to be a good corrosion inhibitor, and this was supplemented by the inhibition efficiency results (73.13%, 71.05% and 89.50%) obtained through weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy at 50 x 10-5 M, respectively. In addition, inhibition efficiency increased with the increase in the glycerol stearate. The rate of corrosion rate ( ), current density was observed to reduce and charge transfer resistance was observed to increase in the presence of the increasing glycerol stearate concentrations. Furthermore, from weight loss measurements, the inhibition efficiency values were observed to decrease with the increase in temperature. Glycerol stearate effectively prevented corrosion by adsorbing to the metal surface and this was confirmed by Fourier transform infrared spectroscopy. Negative values of the free Gibbs energy demonstrated a spontaneous corrosion process. The morphology of the metal surface of the pristine metal, metal in corrosive medium and metal in the presence of the inhibitor were varied with the aid of the scanning electron microscopy. The adsorption of glycerol stearate on the metal surface was found to follow the Langmuir adsorption isotherm model.
This work was financially supported by the National Research Foundation (NRF) and the Sasol foundation. University of Limpopo, South Africa. Dr. T Pesha would also love to acknowledge Mintek, Dr. A. N. Jijana, Dr. P Mkhonto and Mr. T.G. Tsoeunyane at the University of Johannesburg (Doornfontein) for helping with electrochemical experiments.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

©2023 Pesha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.