Journal of
eISSN: 2473-0831


Research Article Volume 3 Issue 3
Correspondence: Narotam Sharma, Central Molecular Research Laboratory, Department of Biochemistry, Shri Guru Ram Rai Institute of Medical & Health Sciences, Patel Nagar, Dehradun-248001, Uttarakhand, India
Received: October 17, 2016 | Published: October 24, 2016
Citation: Arora B, Agarwal U, Shankar U, Kanojia A, Sharma G, et al. (2016) High Risk Human Papilloma Virus (HR-HPV) Genotyping in Female with Abnormal Cervix. J Anal Pharm Res 3(3): 00059. DOI: 10.15406/japlr.2016.03.00059
Human Papilloma Virus (HPV) infection is transmitted primarily via sexual and affects mostly basal epithelial cells of the skin or inner lining of tissues and are categorized as cutaneous types or mucosal types. It is known that HPV infection is strongly related to the development of cervical cancer, as viral DNA is detected in 99% of cervical cancer cases worldwide. Proposed study includes 14 cervical brushing, collected from females with cytologically abnormal cervix. The target genes E6 and E7 were amplified by Conventional PCR. Three cases with High grade squamous intraepithelial lesion (HSIL) came positive. Further the positive cases were digested by restriction endonucleases enzymes for RFLP and the amplicon size for high risk HPV type 16 and type 18 were obtained at 452bp and 455bp respectively. Three cases came positive, out of which HPV type 16 came in two cases with one HPV type 18. High risk HPV characterization at an early stage will prevent further progression of the disease.
Keywords: oncogeni, cytologically; endonucleases, amplicon, DNA, venereal diseases, trichomoniasis, syphilis, gonorrhea, chlamydia, abnormal cervix
Oncogenic, Restriction endonucleases, Venereal Diseases, Cutaneous, Abnormal cervix Sexually transmitted infections (STIs), and Venereal Diseases (VD), commonly spread by sexual activities, especially vaginal intercourse, anal sex and oral sex.1 Most STIs do not cause symptoms. More than 30 different bacteria, viruses, and parasites can cause STIs. Bacterial STIs includes Chlamydia, gonorrhea, and syphilis among others. Each year, there are an estimated 357 million new infections with 1 of 4 STIs: chlamydia, gonorrhoea, syphilis and trichomoniasis.2,3 More than 500 million people are estimated to have genital infections with herpes simplex virus (HSV). More than 290 million women have a human papillomavirus (HPV) infection.4–6 Drug resistance, especially for gonorrhea, is a major threat to reducing the impact of STIs worldwide. Viral STIs includes genital herpes, Human Immunodeficiency Virus /AIDS and Human Papilloma Virus (HPV) and genital warts among others.7,8 HPV is a small, non-enveloped and double stranded DNA virus with a genome of approximately 8,000 nucleotides and with the diameter of 55nm. It establishes infection on basal epithelial cells of the skin or inner lining of tissues and is categorized as cutaneous types or mucosal types. More than 110 genotypes have been cataloged and sexually transmitted. Thus in the current work Human Papilloma Virus Genotypes were Characterized by Restriction Fragment Length Polymorphism (RFLP) Polymerase Chain Reaction (PCR) in females with Abnormal Cervix.
Clinical specimens mainly cervical brushings were collected from females with abnormal cervix in virus transport medium. Further the DNA was extracted from all the specimens. Selection of the target HPV DNA sequences has been based on the study of highly conserved regions in the HPV genome for tested generic genotypes (GEN1 and GEN2 primers), and of minimally variable regions for tested oncogenic HPV genotypes (ONC1 and ONC2). Selected regions have a high degree of conservation between the tested genotypes. Pair 1 (GEN1 and GEN2) defines a segment of approximately 400 bp, while Pair 2 (ONC1 and ONC2) defines a sequence of approximately 250 bp in oncogenic HPV genotypes. The master mix was prepared for HPV oncogenes, mainly early genes designated as E6 and E7. Amplification was done for these target genes. Amplification was done by setting the cycling parameters as; initial duration for 94ºC for 5 minutes, followed by 35 repetitive cycles of denaturation at 94ºC for 30seconds, annealing at 55ºC for 1 min, followed by extension at 72ºC for 1 min. Final extension was given at 72ºC for 10 minutes. We used 3B Black biotech kit for the amplification of E-6 and E-7 genes of HPV genome. Aliquot 40μl of the reaction mixture in each amplification vial from the kit. Add 50-100ng DNA from the purified samples to each reaction vial. Complete up to 50μl final volume with sterile distilled water. RFLP Analysis: Amplification products are subjected to a restriction digestion using five different restriction endonucleases. The obtained patterns indicate the presence of oncogenic HPV genotypes. For RFLP analysis we performed five enzymatic digestions for each amplification. Prepared each Digestion mixture following the indications as: Digestion A: μl Enzyme A + 1μl Buffer A, Digestion B: 1μl Enzyme B + 1μl Buffer B, Digestion C: 1μl Enzyme C + 1μl Buffer C, Digestion D: 1μl Enzyme D + 1μl Buffer D/E, Digestion E: 1μl Enzyme E + 1μl Buffer D/E. Detection of digested products is performed by agarose gel electrophoresis followed by ethidium bromide staining. 3B biotypap kit. In agarose gel electrophoresis, pior to RFLP, the presence of HPV is indicated by a band of approximately 450 bp. If oncogenic HPV genotypes are present in the sample, an additional band of 250 bp will also appear.
A total of 14 specimens were collected from females with cytologically abnormal cervix. The specimens were collected from Shri Mahant Indiresh Hospital which includes Gynecology Department and further they were processed and their DNA was isolated by silica column method. The target genes E6 and E7 were amplified by PCR. Amplicon size of 252bp was obtained for Oncogenic HPV. Further the positive cases were digested by restriction endonucleases enzymes for RFLP and the amplicon size for high risk HPV type 16 and type 18 were obtained at 452bp and 455bp respectively. Three cases came positive, out of which HPV type 16 came in two cases with one HPV type 18.
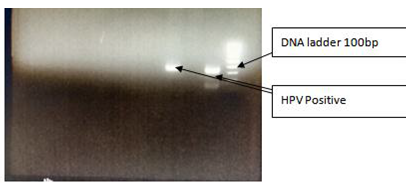
Figure 1 Gel picture depicting the band of oncogene (252 bp amplicon size).
Well 1: and Well 3: HPV Positive.
Well 2: HPV Negative.
Well 3: DNA Ladder 100bp

Figure 2 Agarose gel electrophoresis RFLP Picture.
Well 1: Restriction Enzyme A + Buffer A- Negative.
Well 2: Restriction Enzyme B + Buffer B –Negative.
Well 3: Restriction Enzyme C + Buffer C –Positive.
Well 4: Restriction Enzyme D + Buffer D/E –Negative.
Well 5: Restriction Enzyme E + Buffer D/E – Negative.
Well 6: DNA Ladder 100bp.
HPV infection is transmitted primarily via sexual activity. Half of all new cases are detected during the first three years of sexual activity.9 Genotyping by PCR-RFLP allows the HPV to be typed, and is easier and less expensive than sequencing. The method is simple and robust, does not require sophisticated equipment, and is particularly suited to settings in which financial resources are limited.10,11 PCR-RFLP shows good discriminatory power by differentiating the virus in High Risk or Low Risk, and it is possible to identify single or multiple infections. In this technique, the amplified DNA is digested by restriction enzymes, resulting in DNA fragments of various lengths. Molecular techniques are most commonly used for HPV testing, and are the gold standard for diagnosing the viral infection. Some of these methods may also be used for investigation of regional and country-based type specific HPV prevalence.
Cell infection by HPV is shown by changes in function or in host gene expression, and the detection of these changes may play a major role in the screening and follow-up of infected patients. In spite of their value, molecular techniques still must become more rapid, automated, and low-cost to be of practical use in low-income population and countries. Present study came up with the presence of HPV type 16 in the females with abnormal cervix along with the HPV type 18. The study was limited to the 14 numbers of cases and can be further done for more findings.
The authors are grateful to Honorable Chairman, Shri Guru Ram Rai Education Mission for his kind support and guidance.
The authors declare no conflict of interest.
None.

©2016 Arora, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.