Journal of
eISSN: 2473-0831


Research Article Volume 8 Issue 3
Department of Pharmacology, Faculty of Basic Medical Sciences, University of Port Harcourt, Nigeria
Correspondence: Elias Adikwu, Department of Pharmacology, Faculty of Basic Medical Sciences, University of Port Harcourt, Choba, Rivers State, Nigeria
Received: April 04, 2019 | Published: May 13, 2019
Citation: Adikwu E, Nelson B, Obianime WA. Hepatic morphological alterations in lopinavir/ritonavir–intoxicated rats were abrogated by melatonin and ? lipoic acid. J Anal Pharm Res. 2019;8(3):112-117. DOI: 10.15406/japlr.2019.08.00324
Lopinavir/ritonavir (LPV/r) is an essential component of standard regimens required for the management of human immuno deficiency virus (HIV) infection. However, the clinical uses of LPV/r containing antiretroviral regimens have been characterised by hepatotoxicity, which may involve hepatic morphological alterations. This study assessed the protective effects of melatonin (MT) and alpha lipoic (ALA) acid on hepatic morphological alterations due to lopinavir/ritonavir (LPV/r) in rats. Rats were treated daily by gavage with LPV/r (22.9/5.71, 45.6/11.4 and 91.4/22.9mg/kg) for 90 days. Rats were pretreated daily by gavage with MT (10mg/kg), ALA (10mg/kg) and MT+ALA prior to treatment with LPV/r for 90 days. At the termination of drug administration, rats were weighed and anesthetised. Liver was excised, weighed and evaluated for pathology and biochemical parameters. Treatment with LPV/r significantly (p<0.01) decreased body weight whereas absolute and relative liver weights were increased in a dose–dependent manner in relation to control. Significant (p<0.001) increases in aspartate aminotransferase, alkaline phosphatase, alanine aminotransferase, gamma glutamyl transferase and lactate dehydrogenase levels in a dose-dependent fashion were observed in LPV/r–treated rats when compared to control. Furthermore, hepatic alterations characterised by severe hepatocyte necroses were observed in LPV/r–treated rats. The aforementioned hepatic changes due to LPV/r–intoxication were significantly abrogated in MT (p<0.01), ALA (p<0.01) and MT+ALA (p<0.001) pretreated rats when compared to LPV/r–treated rats. The findings in this study showed that melatonin and alpha lipoic acid can prevent hepatic alterations associated with lopinavir/ritonavir.
Keywords: lopinavir/ritonavir, liver, histology, antioxidants, rats
Protease inhibitors (PIs) are essential components of standard regimens required for the management of human immuno deficiency virus (HIV) infection. However, the clinical use of PIs containing antiretroviral regimens has been characterised by adverse effects such as hepatotoxicity. Therefore, the US FDA drug information contains essential information on possible hepatitis, hepatic failure and death that may characterise the use of PIs.1 Among PI–containing regimes, an average incidence of 10% hepatotoxicity was observed with lopinavir/ritonavir (LPV/r) containing regimens.1 Hepatotoxicity associated with LPV/r is often characterised by altered hepatic serum clinical chemistry, which aggravates with length of therapy.2 Also, perturbations in hepatic morphology characterised by extensive necrosis, ballooning of hepatocytes, distortion in the radial arrangements of hepatocytes and sinusoids were observed in animals.3 Furthermore, hepatic aberrations, marked by hepatocyte necrosis, sinusoidal degeneration, mitochondrial dysfunction, steatosis, and occlusion of sinusoidal lumina were reported in humans.4 In addition, reported hepatic alteration due to LPV/r may involve oxidative stress (OS), nitrosative stress (NS) and lipid peroxidation (LPO) as reported in studies with animals.5
Melatonin (MT), which is an indolamine produced by the pineal gland, is a potent anti–inflammatory and antioxidant agent. It can inhibit free radical–induced OS, NS and LPO.6 MT has an amphillic nature and a small molecular size that allows it to enter both aqueous and lipid environments of cells. In addition to its antioxidant and anti–inflammatory activities, it has the ability to regulate the functions of the immune system. Studies have shown that it is effective against pathological conditions associated with free radicals.7 One of the essential activities of MT is its ability to safeguard and preserve the morphology of tissues and organs from free–radical–induced damage.8 Studies using animals have reported its ability to safeguard the liver against xenobiotic–induced hepatic morphological alterations such as necrosis, cellular degeneration and cell apoptosis.8 Also, it has protected the liver from morphologic perturbations characterised by hepatic dystrophy, fibrosis, haemorrhage, and steatosis in xenobiotic–intoxicated rats.8,9
Alpha–lipoic acid (ALA) is a natural molecule that has a five–membered cyclic disulphide and hydrocarbon tail ending with a carboxylic acid group.10 It functions as a cofactor in multienzyme complexes that catalyse the oxidative decarboxylation of α–keto acid.11,12 It is a universal antioxidant that scavenges and neutralises free radicals. It acts in water and fat–soluble tissues in both its reduced and oxidized forms to inhibit OS and LPO. ALA can facilitate the activities of antioxidants such as vitamin C, vitamin E and glutathione (GSH).13Its anti–inflammatory effect includes modulation of cellular genes expression essential for cytokine production, inflammation, and apoptosis.14Studies using animals have reported the beneficial effects of ALA against various pathologies that may affect the morphology of tissues and organs including the liver.15 It has abrogated xenobiotic–induced fibrosis, necrosis, and steatosis in rats.16-18 In the light of the aforementioned information, the present study examined the protective effects of MT and ALA against LPV/r- induced hepatic alterations in rats.
Drugs and chemicals
LPV/r (Myland Laboratories Limited India), MT and ALA (Shijiazhuang AO Pharm Import and Export Co Ltd China). All other chemicals used for this study are of analytical grade. LPV/r (22.9/5.71, 45.6/11.4 and 91.4/22.9mg/kg), which represents 2, 4 and 8 times the clinical dose,19 MT (10mg/kg) and ALA (10mg/kg) were used for this study.20,21
Experimental protocol
Rats used for this study were divided into 6 groups (A–F). Group A (control) was divided into two sub–groups A1 (placebo control) and A2 (solvent control) of 5 rats each. Rats in groups A1 and A2 were orally treated with normal saline and 1% ethanol respectively for 90 days. Groups B–F contained 15 rats each, which were divided into 3 sub–groups of 5 rats each. Rats in group B were orally treated with MT (10mg/kg), ALA (10mg/kg) and MT+ALA for 90 days. Rats in group C were orally treated with 22.9/5.71, 45.6/11.4 and 91.4/22.9mg/kg of LPV/r for 90 days whereas rats in groups D–F were orally pretreated with MT, ALA and MT+ ALA prior to treatment with LPV/r (22.9/5.71, 45.6/11.4 and 91.4/22.9mg/kg) for 90 days, respectively.
Evaluation of liver biochemical parameters
Rats were anesthetised with diethyl ether, and liver was excised and homogenised. The homogenate was centrifuged, and the supernatant was extracted and evaluated for aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), and lactate dehydrogenase (LDH) using standard test kits according to manufacturer’s specifications.
Histology of the liver
Liver was cut into a slab of 0.5 cm thick and fixed in Bouin's fluid for 24 hours, at which point it was then dehydrated with 70% alcohol. Liver tissues were passed through 90% alcohol and chloroform at different times and embedded in moistened paraffin wax. Serial sections were cut at 5 microns. Liver tissue slides were prepared and wax was removed with the aid of absolute alcohol. The slides were then stained with hematoxylin and eosin. Light microscopic examinations of the slides were performed and relevant sections were photographed.
Statistical method
Results are expressed as mean±SEM. Differences between means were tested for statistical significance using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. P–values<0.05;0.01;0.001 were considered statistically significant.
Measurement of animal growth is routinely used in toxicology studies in order to interpret xenobiotic–related effect on growth. Growth data based on body weight is collected, analyzed, and interpreted for most rodent toxicology studies.22 In the current study, at the end of drug administration, significant (p<0.01) decreases in body weights in a dose-dependent manner were observed in rats treated with LPV/r in relation to control. However, body weights were significantly restored in MT (p<0.05) and ALA (p<0.05) pretreated rats in relation to LPV/r–treated rats (Table 1). The restored body weight was most significant (P<0.01) in rats pretreated with MT+ALA when compared to LPV/r–treated rats (Table 1). The assessment of liver body weight ratio is a better approach to determine changes in liver weight than the assessment of liver weight alone because liver weight largely depends on the body weight.23 The present study observed significant (P<0.01) increases in absolute and relative liver weights in a dose–dependent fashion in LPV/r–treated rats when compared to control (Table 2 & 3). The observed increases in absolute and relative liver weights signified hepatic lesions and liver injury due to intoxication with LPV/r. This may be due to the hepatic stimulation of inflammatory processes by LPV/r.24 However, relative and absolute liver weights were significantly restored in MT (P<0.05) and ALA (P<0.05) pretreated rats when compared to LPV/r–treated rats. Absolute and relative liver weights were best and significantly (P<0.001) restored in rats pretreated with MT+ALA when compared to LPV/r–treated rats (Table 2 & 3). The liver contains AST, ALT, ALP, GGT and LDH. The hepatic content of the aforementioned parameters can be altered with the advent of hepatic morphological alterations caused by to xenobiotics.25 In the present study, treatment with LPV/r caused significant (p<0.001) elevations in liver levels of AST, ALT, ALP, GGT and LDH in a dose-dependent fashion when compared to control (Table 4). The observation attests to hepatic damage caused by treatment with LPV/r. However, the aforementioned parameters were significantly decreased in rats pretreated with MT (p<0.01), ALA (p<0.01) and MT + ALA (p<0.001) in relation to LPV/r–treated rats (Table 4).
Treatment |
Initial body |
Final body |
Absolute liver |
Relative |
weight (g) |
weight (g) |
weight(g) |
liver weight (%) |
|
Control |
221.4±14.0 |
330.4±12.1 |
6.26±0.15 |
1.90±0.13 |
MT |
225.2±10.6 |
340.8±13.2 |
6.41±0.33 |
1.88±0.12 |
ALA |
220.4±13.3 |
335.6±14.6 |
6.48±0.41 |
1.93±0.67 |
MT+ALA |
230.6±15.7 |
350.4±12.4 |
6.50±0.22 |
1.86±0.54 |
Table 1 Effects of melatonin and alpha lipoic acid on body and liver weights of albino rats
Values are expressed as Mean± SEM, MT, Melatonin; ALA, Alpha lipoic acid; n=5.
|
LPV/r |
MT+LPV/r |
ALA+LPV/r |
MT+ALA +LPV/r |
Dose (mg/kg) |
FBW (g) |
FBW (g) |
FBW (g) |
FBW (g) |
Control |
330.4±12.0 |
340.7±15.1 |
340.6±14.2 |
340.5±15.1 |
22.9/5.71 |
300.2±13.1 |
320.0±12.7 |
310.0±11.4 |
320.6±12.6 |
45.6/11.4 |
150.4±11.5* |
210.4±13.3** |
200.7±14.7** |
300.3±16.3*** |
91.4/22.9 |
120.6±11.8* |
180.7±11.2** |
175.4±13.5** |
280.2±10.0*** |
Table 2 Effects of melatonin, alpha lipoic acid and lopinavir/ritonavir on body weight of albino rats
Values are expressed as Mean ± SEM, MT, Melatonin; ALA, Alpha lipoic acid; LPV/r, Lopinavir/ritonavair; n=5.
*Significant (P<0.01) difference when compared to control
**Significant (P<0.05) difference when compared to LPV/r
***Significant (P<0.01) difference when compared to LPV/r
|
LPV/r |
|
MT |
|
ALA |
|
MT+ALA |
|
Dose (mg/kg) |
ALW (g) |
RLW (%) |
ALW(g) |
RLW (%) |
ALW (g) |
RLW (%) |
ALW (g) |
RLW (%) |
Control |
6.26±0.15 |
1.84±0.13 |
6.26±0.15 |
1.84±0.13 |
6.26±0.15 |
1.84±0.13 |
6.26±0.15 |
1.84±0.13 |
22.9/5.71 |
6.55±0.76 |
2.18±0.47 |
6.40±0.26 |
2.00±0.67 |
6.43±0.13 |
2.07±0.16 |
6.40±0.43 |
2.00±0.54 |
45.6/11.4 |
8.89±0.37* |
4.23±0.59* |
6.45±0.23** |
2.22±0.41** |
6.55±0.02** |
2.29±0.57** |
6.26±0.19** |
2.07±0.37** |
91.4/22.9 |
10.9±0.17* |
7.23±0.57* |
8.50±0.10*** |
4.03±0.86*** |
8.99±0.10*** |
4.49±0.63*** |
6.24±0.33*** |
2.23±0.19*** |
Table 3 Effects of melatonin, alpha lipoic acid and lopinavir/ritonavir on liver weight of albino rats
Values are expressed as Mean ± SEM, MT, Melatonin; ALA, Alpha lipoic acid; LPV/r, Lopinavir/ritonavair; ALW, Absolute liver weight; RLW, Relative liver weight; n=5.
*Significant (P<0.01) difference when compared to control
**Significant (P<0.05) difference when compared to LPV/r
***Significant (P<0.01) difference when compared to LPV/r
Group |
AST |
ALT |
ALP |
GGT |
LDH |
U/L |
U/L |
U/L |
U/L |
U/L |
|
Control (placebo) |
255.7±16.7 |
230.6±15.9 |
250.1±20.4 |
25.9±3.00 |
240.5±13.6 |
MT |
245.0±18.3 |
227.4±17.0 |
245.9±21.3 |
24.7±3.53 |
238.6±12.0 |
ALA |
247.9±15.2 |
225.4±13.2 |
240.6±22.8 |
24.2±3.30 |
253.2±14.7 |
MT+ALA |
240.4±13.5 |
220.1±18.7 |
235.2±20.8 |
22.5±4.45 |
230.7±13.9 |
LPV/r (91.4/22.9mg/kg) |
860.3±15.7* |
799.9±14.6* |
959.3±40.6* |
798.0±6.01* |
834.1±24.2* |
MT+ LPV/r |
521.0±14.0** |
410.8±16.5** |
500.0±32.7** |
40.4±7.53** |
400.3±13.5** |
ALA+ LPV/r |
563.5±17.3** |
450.7±17.3** |
561.3±31.4** |
43.6±5.27** |
422.9±19.5** |
MT+ ALA+LPV/r |
266.6±16.4*** |
241.7±18.7*** |
269.4±19.9*** |
27.3±4.43*** |
247.6±15.7*** |
Table 4 Effects of melatonin, alpha lipoic acid and lopinavir/ritonavir on biochemical parameters in the liver of albino rats
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; GGT, Gamma glutamyl transferase; LDH, Lactate dehydrogenase; MT, Melatonin; ALA, Alpha lipoic acid; LPV/r, Lopinavir/ritonavair; n=5. Values are expressed as M±SEM,
*Significant (P<0.001) difference when compared to control
**Significant (P<0.01) difference when compared to LPV/r
***Significant (P<0.001) difference when compared to LPV/r
Histopathology is a critical part of the toxicologic and risk assessment of foods, drugs, chemicals, biologics, and medical devices. Histopathology is the study of the structural manifestations of disease at the light–microscopic level. Therefore, histopathology is necessarily a largely confirmative, descriptive and interpretive science. In addition to other applications, it can establish correlations between perturbations in serum biomarkers with morphologic alterations that may occur at tissue or organ levels due to a test substance.26 In the current study, histological assessment of the liver of control rats showed normal histology (Figure 1A). Also, the liver of rats treated with MT and ALA showed normal histology (Figure 1B, D). On the other hand, extensive hepatocyte necrosis was observed in the liver of LPV/r–treated rat (Figure1E), which is consistent with previous report.27 However, the liver of rats pretreated with MT and ALA showed absence of hepatocyte necroses (Figure 1G, H). This observation connotes that MT and ALA abrogate LPV/r–induced hepatic structural changes in pretreated rats. The protective effect of ALA observed in this study can be correlated with the study by Abdel Zaher et al.28 which reported that ALA restored liver structure in acetaminophen–intoxicated rats.28 Also, findings in the present study can be correlated with the reported protective effect of MT against cadmium–induced hepatic morphological alteration in rats.29 The mechanism by which LPV/r causes hepatic morphological alteration is not clear; however, it may involve OS, LPO and the stimulation of inflammatory cascade. Previous studies have reported hepatic OS leading to LPO in LPV/r–treated rats.30 OS is associated with oxidative radical production, which can result in histopathological lesions with a broad spectrum, ranging from hepatotoxity to hepatocellular carcinoma in an orchestrated manner. Evidence have shown that increased free radical concentration and activity are commonly observed during hepatic cell damage characterised by OS and LPO.31 LPO is a complex process that involves the formation and propagation of lipid radicals, the uptake of oxygen, rearrangement of double bonds in unsaturated lipids and the eventual destruction of membrane lipids.32 Exposure of hepatic proteins to reactive oxygen species can produce denaturation, loss of function, cross–linking, aggregation and fragmentation of connective tissues.33 In the current study, the protective effects of MT and ALA may be due to their free radical scavenging activities, inhibition of OS, LPO, and inflammation. Also, MT and ALA might have increased endogenous antioxidants activities and stabilized hepatic plasma membrane. ALA is a powerful antioxidant due to its ability to scavenge free radicals in both lipophilic and hydrophilic environments and to regenerate endogenous antioxidants. Also, it can inhibit LPO, OS, and prevent protein glycation and repair biomolecules damaged by oxidation.34,35 MT is an amphiphilic molecule that can freely cross cell membranes and enter cells where it has been reported to alter redox balance by increasing antioxidant synthesis, activity and scavenges free radicals. It can inhibit LPO thereby preventing DNA, lipid, and protein damage.36 Furthermore, experimental evidence showed that MT and ALA can inhibit inflammation–induced hepatotoxicity by up regulating anti–inflammatory mediators and inhibiting pro–inflammatory mediators.37,38
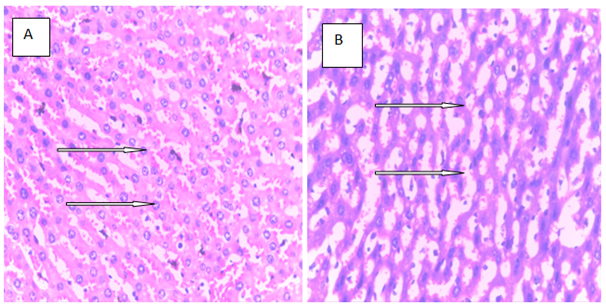
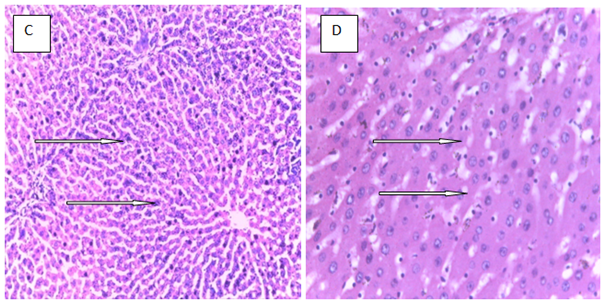
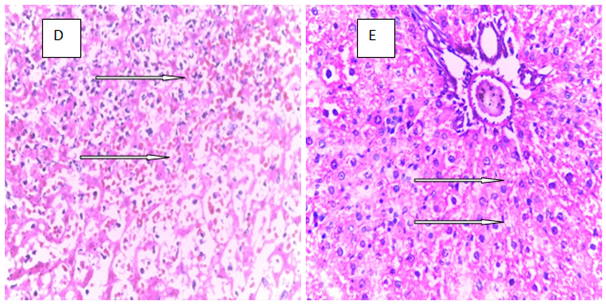
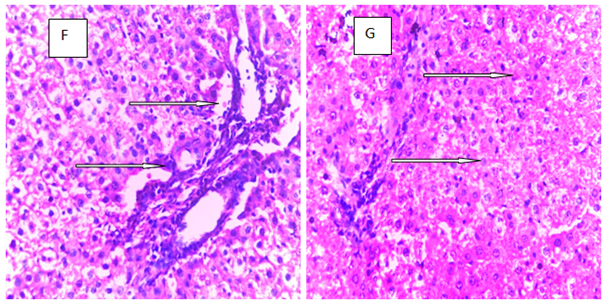
Figure 1 The above figures (A–G) are micrographs of the Hand E stained sections of the liver of control rat, and the liver of rats in the experimental groups. Normal hepatocytes were observed in the liver of control rat Figure 1A. Also, normal hepatocytes were observed in the liver of rats treated with melatonin, alphalipoic and a combination of melatonin, and alphalipoic (Figures 1E). On the other hand, extensive hepatocyte necrosis was observed in the liver of lopinavir/ritonavir-treated rat Figure 1E. However, normal hepatocytes were observed in rats pretreated with individual dose of melatonin and alpha lipoic acid respectively Figure 1F and G. Also, normal hepatocyte was observed in the liver of rats pretreated with a combination of melatonin and alpha lipoic (Figure 1H).
The current study showed that melatonin and alpha lipoic acid can abrogate alterations in hepatic morphology that may arise with the use of lopinavir/ritonavir.
None.
The authors declare that there is no conflict of interests.

©2019 Adikwu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.