Journal of
eISSN: 2473-0831


Review Article Volume 4 Issue 4
Correspondence: Satya P Gupta, Department of Applied Sciences, National Institute of Technical Teacher?s Training and Research (NITTTR), Bhopal 462002, India
Received: February 27, 2017 | Published: April 4, 2017
Citation: Sharma A, Gupta SP, Siddiqui AA, Sharma N (2017) HCV NS3/4A Protease and its Emerging Inhibitors. J Anal Pharm Res 4(4): 00108. DOI: 10.15406/japlr.2017.04.00108
Globally, Hepatitis C virus (HCV) is a leading cause of chronic liver disease, hepatocellular carcinoma and cirrhosis and the most common reason for liver transplantation. Presently, the most common treatment for HCV infected patients is the combinations of pegylated interferon and ribavirin that provide a sustained response in patients, but its side effects are brutal. Therefore, attempts have been made to target the two important enzymes, NS3 protease and NS5B RNA polymerase, that are crucial for the replication of HCV. The article presents the development of some NS3/4A protease inhibitors that have reached the final stages of clinical trials.
Keywords hepatitis C virus, ns3/4a protease, macrocyclic inhibitors, linear inhibitors
HCV, hepatitis c virus; RdRp, RNA dependent RNA polymerase; NS, nonstructural; TC-PTP, T cell protein tyrosine phosphatase; MAVS, mitochondrial antiviral signaling protein
Hepatitis C virus (HCV) belongs to the family of Flaviviridae. This family also includes other viruses responsible for severe human diseases, such as yellow fever virus, dengue virus, St. Louis encephalitis virus, and West Nile virus. About 170 million people, estimating 3% of the global population are infected with HCV. Chronic HCV infection may lead to progressive liver injury, cirrhosis and in some cases hepatocellular carcinoma.1s Hepatitis C virus is an envelope, positive single stranded RNA. Its genome is composed of 9.5kb and encodes a 3000 amino acid sequence residue polyprotein.2 For HCV entry, human CD81 receptor is required which binds directly with E2 protein of HCV. This CD81 receptor is a widely distributed cell-surface tetraspanin that participates in the formation of molecular complexes on various cell types like hepatocytes, natural killer cells, B-lymphocytes, and T-lymphocytes. Thus, the HCV not only exploits hepatocytes but also modulates the host immune response.
The HCV has principally two drug targets, its RNA polymerase and the protease enzymes. where the former catalyzes the RNA relication. This RNA-dependent RNA-polymerase (RdRp), however, lacks a proof reading activity which leads to a high mutation rate and a large number of mutations.3 The HCV encodes a polyprotein of approximately 3000 amino acids,4 which is cleaved cotranslationally and post-translationally to produce four structural proteins (C, E1, E2, and p7) and six nonstructural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B).5-7 Only the NS3–NS5B region of the polyprotein is required for genome replication in cell culture. NS3-NS4A, in which NS4A acts as a cofactor, is a serine protease belonging to trypsin/chemotrypsin superfamily. NS4A directs the localization of NS3 and modulates its enzyme activity.8 NS3-NS4A is simply written as NS3/4A. It plays a critical role in producing the important components for viral replication by cleaving the scissile bond between NS3-NS4A. Based on the available structure of HCV protease, there are three possible sites for drug design, namely zinc binding site, NS4A binding groove, and substrate binding site, of which the last one has been found to be more promising. However, the design and development of NS3/4A inhibitors is more challenging than the design of inhibitors of other proteases such as thrombin and elastase, because the binding pocket of NS3/4A is more solvent exposable, shallow and hydrophobic than that of other proteases.
Proteases often have a specific recognition site where the peptide bond in substrate is cleaved. As shown in Figure 1, there is a general nomenclature of the cleavage site positions in the substrate which were given by Schechter and Berger.9,10 In the figure, a ‘P’ refers to a amino acid residue in the peptide substrate. The cleavage site is referred to by P1-P1ʹ. A Pn refers to a residue on N-terminal direction and a Pnʹ to a residue on the carboxyl side. The corresponding binding sites in enzyme are designated by Sn and Snʹ. This review describes the structure and functions of NS3/4A protease and discusses a wide variety of HCV NS3/4A protease inhibitors that have been critical in the evolution of therapies for Hepatitis C.

Figure 1 Nomenclature of amino acid residues in peptide substrate. A Pn refers to a residue on N-terminal direction and a Pnʹ to a residue on the carboxyl side.
NS3/4A Protease: Structure and Function
Structural studies have confirmed that NS3 protease is a chymotrypsin like serine protease.9 where Ser139, His57, and Asp81 in HCV NS3 form the catalytic triad of the enzyme (Figure 2). The crystal structure and specificity of HCV protease have been characterized in detail.9-11 Its catalytic triad has been found to be constituted of Ser139, His57, and Asp81 residues (Figure 2). The protease contains two β barrels and a 30-residue extension at the N-terminus. The C-terminus which is two-third of NS3 has helicase activity and is located in the active site of NS3 protease domain. Helicases are enzymes that bind and may even remodel nucleic acid or nucleic acid protein complexes. The zinc ion also plays a vital role in the stabilization of the enzyme structure and is coordinated by three cysteine residues (Cys97, Cys99, and Cys145) and a histidine (His149) residue (Figure 3). It is located on the opposite side of the protease from the active site. The P1 amino acid cysteine and P1ʹ serine residue are conserved in all the enzyme substrates. The S1 pocket is hydrophobic, containing Phe154, Ile135, and Ala157 residues, where the sulfhydryl group of cysteine from the substrate makes an efficient interaction with the aromatic group of Phe-154.
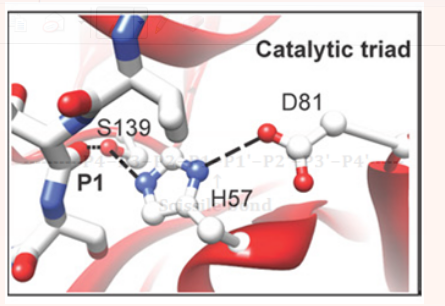
Figure 2 Catalytic triad of NS3 (S139, H57, and D81) in ball and stick model, interacting with a modeled peptide (black dashed lines represent the hydrogen bonds).11 The American Society for Biochemistry and Molecular Biology, Inc.
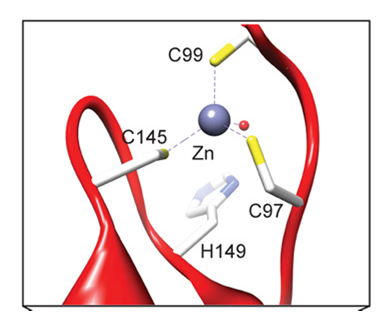
Figure 3 Zinc-binding site in NS3/4A coordinated with C99, C145, C97, and H149, where ‘C’ stands for Cys and ‘H’ for His residues.11 The American Society for Biochemistry and Molecular Biology, Inc.
The NS4A cofactor having 54 residues is required for the cleavage at NS3-NS4A junctions at the polyprotein. The cofactor allows proper NS3 protease folding. Incorporation of NS3/4A complex into the membrane and its binding increases the proteolytic activity 1000 fold.12 NS3-NS4A protease not only hydrolyses the viral proteins but also targets host cell proteins which plays important role in innate immunity and growth factor signaling like MAVS (mitochondrial antiviral signaling protein),13,14 TC-PTP (T-cell protein tyrosine phosphatase),15 and TRIF (TIR domain containing adaptor inducing IFN-β).14 NS4A cofactor is covalently linked to the structure by fusing the C-terminus of NS4A to the N-terminus of NS3. It is a peptide of both hydrophobic N-terminus and hydrophilic C-terminus that leads to the activation of NS3 protease as a cofactor.6,16 Figure 4 shows how NS4A is complexed with NS3 [10a]. In Figure 4 is also shown how two reversible covalent inhibitors, α-ketoacids (a and b), can bind with the protease. Inhibitors span from P1 to P4 residues. Both inhibitors are shown to bind in an extended backbone conformation, forming an antiparallel β-sheet with one enzyme β-strand. The inhibitor ‘b’ is superimposed on ‘a’. The P1 residue has the maximum contribution to the binding energy. P2-P4 side chains are partially solvent exposed. Figure 5 shows how an inhibitor, here a, can form the hydrogen bond with the protease.10
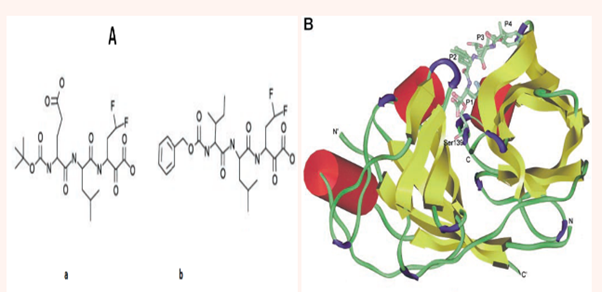
Figure 4 structures of two α-ketoacid inhibitors that are bound in secondary structure of NS3 complexed with cofactor NS4A. Helices are shown in red, sheets in yellow, turns in purple, and random coils in green. N and C indicate the N and C termini of the protease domain, whereas Nʹ and Cʹ indicate the N and C termini of the NS4A cofactor peptide. Inhibitor ‘b’ is superimposed on inhibitor ‘a’ 10a The American Society for Biochemistry and Molecular Biology, Inc.
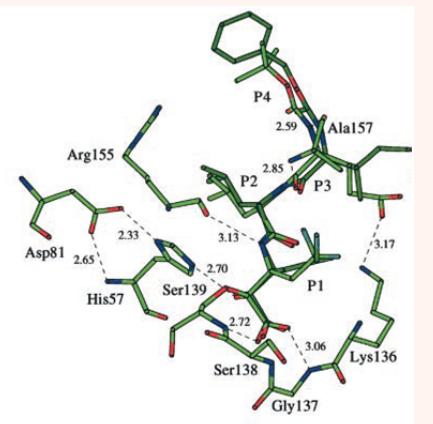
Figure 5 An stereo view of hydrogen bond interactions of inhibitor ‘a’ with the protease [10a]. The American Society for Biochemistry and Molecular Biology, Inc.
Lemke et al.17 studied the binding characteristics of a compound, BI-201335, with NS3/4A. BI-201335 (Figure 6), also known as Faldaprevir, was an experimental NS3/4A inhibitor developed by Boehringer-Ingelheim, which reached phase 3 clinical trials in 2011, but was withdrawn in 2014 because of development of better HCV therapy. A Combined X-ray, NMR, and Kinetic Analyses had revealed that when BI-201335 binds with NS3/4A, it adopts an extended conformation spanning from S1-S4 (Figure 7a) and that its binding site is primarily hydrophobic with discrete polar patches. The extended conformation was found to be paired with an exposed strand of C-terminal β-barrel, forming three substrate-like intermolecular hydrogen bonds (Figure 7b). Figure 7b also showed a water molecule (W1) to be central to acid binding. The study of Lemke et al.17 also indicated the presence of a halogen bond between the bromine of the inhibitor and the backbone carbonyl oxygen of Asp-79 (Figure 7b). A halogen bond involves a noncovalent interaction between a polarizable halogen atom, acting as a Lewis acid, and a Lewis base (oxygen of D79).
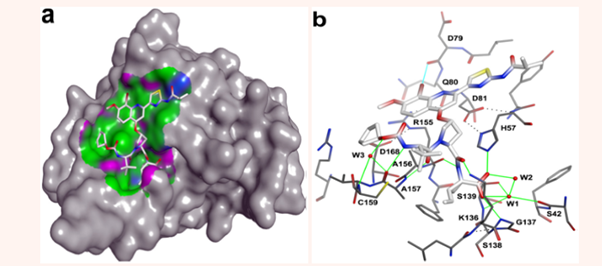
Figure 7 BI-201335 (Faldaprevir) interacting with NS3-NS4A protease. (a) Hydrophobic and hydrogen bond interactions where hydrophobic surface of the protease is shown by green and H-bonding surface by purple. There is a mildly polar region also on the protease surface as shown by blue with which the inhibitor can interact, (b) intermolecular hydrogen and halogen bond interactions as shown by green and cyan lines, respectively. Select intramolecular hydrogen bonds are indicated by black dotted lines.
Urbani et al.18 studied the substrate specificity of NS3 protease and found that the NS3 protease has an absolute requirement for a small residue in the P1 position of the substrate and that the optimization of the P1 binding site occupancy primarily influences transition state binding. If distal binding sites are occupied, they determine both ground and transition state binding. Optimization of contact at distal binding sites may affect synergistically to cleavage efficiency.
Development of NS3/4A Protease Inhibitors
Protease inhibitors are divided into four major classes:
Breakthrough Structures (Peptidic/Peptidomimetic Inhibitors)
As HCV NS3 contains two domains: an N-terminal serine protease and a C-terminal RNA helicase [10b], Boehringer–Ingelheim initially established N-terminal hexapeptide product (DDIVC-OH) of the substrate, derived from the NS5A/5B that inhibited competitively NS3 protease complexed with NS4A cofactor peptide.20 Studies at IRBM were also made to find the competitive inhibitors of NS3.21 This led to the discovery of one of the products of cleavage of the NS4A-NS4B peptide (Ac-DEMEEC-OH). This peptide was modified into a hexamer 2 by various combinatorial and single mutations techniques. Studies revealed that for interaction between peptide inhibitors and NS3 the presence of P1 carboxylic acid was critical.
The first class generation agents were found to be selective against HCV genotype 1 and could be used in association with peg-interferon and ribavirin. They are reversible covalent inhibitors that form covalent bonds with an active site Ser139. The linear ketoamides which gained FDA approval were Boceprevir
P1–P3 macrocycles
The reversible inhibitors are largely sub-classified based on their structure: P1-P3 macrocyclic, acyclic and P2-P4 macrocyclic compounds. A potent P1-P3 non-covalent inhibitor, Ciluprevir (3) (BILN 2061), was discovered at Boehringer–Ingelheim.22 The phase I clinical studies on Ciluprevir showed promising effects but further the clinical development of it was discontinued due to cardiotoxic issues in animals.23 The replacement of the pyrrolidine ring from the proline-containing macrocycle with a cyclopentane surrogate led to a novel series of HCV NS3/4A protease inhibitors with high potency, in which Simeprevir (TMC435350, 4) was found to display low in vitro clearance and high permeability for further preclinical and clinical development as an HCV drug candidate.24
Acyl sulfonamide, P1ʹ interaction (BMS-605339)
Researchers at Bristol-Myers Squibb discovered a tripeptidic inhibitor, BMS-605339 (5), as an inhibitor of NS3/4A by incorporating a cyclopropyl-acyl sulfonamide moiety to improve the potency of carboxylic acid prototype which favors nonbonding interactions within the P1ʹ site of NS3/4A protease.25 The addition of cyclopropyl-acyl sulfonamide showed 50-fold increase in biochemical potency and a >100-fold increase in cell-based/replicon potency. However, BMS-605339 (5) was discontinued from clinical trials due to its cardiovascular side effects, but a new compound, BMS-890068 (6), discovered by Sun et al.26 was found to have better antiviral and pharmacokinetic properties.
P2-P4 macrocycles
Utilizing a different macrocyclization strategy with a linker from P2 to P4, some potent NS3/4A inhibitors were developed.27 These compounds were Vaniprevir (MK-7009, 7),28,29 MK-1220 (8),30 and MK-5172 (9).31These compounds were developed following the development of MK-4519 (10) by Brown et al.32 which was optimized for genotype 1bpotency. The major genotype in the United States is genotype 1, with genotypes 2 and 3 representing sizeable populations as well. Vaniprevir (7) and MK-4519 (10) are quite potent against genotype 1, but are less active against genotype 3a. Within the optimized series, MK-1220 (8) emerged as a candidate suitable for development.30
In addition to these compounds, several other potential NS3/4A inhibitors have been developed which belong to second and third generation research. These compounds are given in Figure 8 and their development and status are described below.
Description of Compounds of Figure 8
Faldaprevir: Was being developed by Boehringer-Ingelheim and reached Phase III clinical trials, but because of availability of better anti-HCV drugs its approval was not pursued.
Due to high variability of Hepatitis C virus (HCV), the standard anti-HCV therapies have failed in HCV-infected patients. Therefore, scientists are trying to find out the suitable alternative therapy for HCV infection. The protease NS3/4A in HCV has been found be an attractive target for this. As a result, so far several potential NS3/4A inhibitors, like Ciluprevir (BILN 2061) (3) Simeprevir (TMC435350) (4), and many others as given in Figure 8 have been developed. Efforts are being made to find still better NS3/4A inhibitors.
None.
The authors do not have any personal or financial interests.
None.

©2017 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.