Journal of
eISSN: 2473-0831


Short Communication Volume 13 Issue 1
Faculty of Pharmaceutical Science, Kobe Gakuin University, Japan
Correspondence: Yamahara Hiroshi, Professor, Faculty of Pharmaceutical Science, Kobe Gakuin University, Kobe, Hyogo, Japan
Received: January 04, 2024 | Published: January 18, 2024
Citation: Hiroyuki H, Hiroshi Y. Evaluation of single granule release characteristics of controlled release granules with porous ethylcellulose membrane. J Anal Pharm Res. 2024;13(1):1-3. DOI: 10.15406/japlr.2024.13.00431
A basic dissolution study of single granule in a controlled-release capsule formulations was conducted. Controlled-release granules were prepared using porous Ethylcellulose membranes, and single granule dissolution profiles was compared with those of the Japanese pharmacopoeia (JP) paddle method. Dissolution profile from one granule was found to be dependent on the solubility of the drug.
Keywords: dissolution test, single granule, theophylline, diltiazem
Multiple-unit dosage forms such as fine granules and granules are effective controlled-release dosage forms for achieving sustained blood concentration transitions because of their small changes in bioavailability and low risk of local irritation due to small variations in gastric residence time and gastrointestinal migration.1 Known drug release control techniques include a method of controlling drug release by controlling passive diffusion of the drug in a matrix, and a method of controlling drug release by coating with a release control membrane.2 The release of drug from a matrix is expected to show little variation if the matrix is homogeneous. The drug release from particles coated with a controlled-release membrane varies depending on both the thickness of the membrane and the uniformity of the film, on the other hand, it is important to establish a technique to uniformly coat the controlled-release membrane. Dissolution test method listed in the Pharmacopoeia is an established method for evaluating drug release and plays an important role in predicting how a drug product will act in vivo, however, it is not possible to detect variation among multiple particles in a multiple-unit formulation. Therefore, we attempted to establish a release test for each granule in order to construct a system that can measure the release characteristics of each granule in a multiple unit preparation. For the dissolution test for single granule, volume of dissolution medium was set to 10 mL as a preliminary method because the drug concentration would be too low to detect it in 900 mL of dissolution medium. Theophylline and Diltiazem hydrochloride with different solubilities were used as model drugs. For adding controlled release ability to drug contained granules, a porous Ethylcellulose membrane coating technique using CF-granulator3 was conducted.
Chemicals
Theophylline was purchased from Katayama Chemical Industry (Osaka, Japan). Nonpareil (NP, NP103, 32/42 mesh) was purchased from Freund Corporation (Tokyo, Japan). Ethylcellulose (EC, #10) and polyvinylpyrrolidone (PVP, Corydon K30) were purchased from Dow Chemical (USA) and BASF (Germany), respectively. All other reagents were used special grade products commercially available.
Preparation of porosity-controlled ethylcellulose film coated beads
Drug-loaded uncoated beads were prepared by layering the drug powder using a CF-granulator (CF-360EX, Freund Industrial Co., Ltd.). The mixed-powder of Theophylline or Diltiazem hydrochloride (1000 g) and sucrose (600 g) was pulverized by a hammer mill (Sample mill, Dalton Co., Ltd., Tokyo, Japan), and they were slowly applied to the Nonpareil1 seeds (1000 g) while continuously spraying the binding agent solution (20 w/w% aqueous solution of sucrose) to obtain the drug-loaded beads. The standard operating conditions applied were as follows: spray solution feed, 2–7 mL/min; spray air pressure, 0.8 kg/cm2; blower rate, 150–250 L/min; blower temperature, 60 deg; and rotating speed, 150 rpm. The uncoated beads were oven-dried for 16 h at 45 deg. After drying, the beads were sieved (no. 22 sieve, <0.7 mm) to remove both the agglomerate and the fine particles.
The uncoated beads of Theophylline or Diltiazem hydrochloride were then film-coated by spraying an aqueous ethanolic solution of EC with the CF-granulator. The formula of porous EC-coated granules of Theophylline and Diltiazem hydrochloride are listed in Table 1. The polymeric concentration of the coating solution was 5 w/w%, and the solvent composition of the solution was 80/20 for ethanol/water. The operating conditions of the coating process were as follows: spray solution feed, 6 mL/min; spray air pressure, 1 kg/cm2; blower rate, 100–200 L/min; blower temperature, 50 deg; and rotating speed, 150 rpm. The film-coated beads were oven-dried for 16 h at 45 deg. The size of the granule was approximately 0.8–1 mm in diameter.
|
Theophylline beads |
Diltiazem hydrocholide beads |
|
|
Component |
Quantity(%) |
Quantity(%) |
|
Theophylline |
34 |
- |
|
Diltiazem hydrochloride |
- |
34 |
|
Sucrose (filler) |
20 |
17 |
|
Nonpareil-103a |
34 |
34 |
|
Sucrose (binder)b |
9 |
9 |
|
Ethylcellulose(coating layer)c |
3 |
6 |
Table 1 Composition of theophylline and diltiazem hydrochloride loaded porosity-controlled ethylcellulose film coated granules
aSize: 24–32 mesh
bThe binder solution is 25% aqueous ethanol containing 20% sucrose
cThe coating solution is 85% aqueous ethanol containing 5% ethylcellulose
Dissolution experiments for single granule were performed using glass test tube, containing 10 mL of water at 37 deg with constant stirring at 100 rpm using micro stirrer bar(φ2×7mm). To determine the amount of Theophylline or Diltiazem from the EC-coated granule, 10 uL of the dissolution medium was assayed by HPLC. HPLC analysis was carried out using a Shimadzu LC-4A apparatus equipped with a Spherisorb3 ODS column (4.6mm i.d. 250 mm, Chemco Scientific Co., Ltd., Japan) and a Shimadzu SPD-6A UV monitor at 292 nm for theophylline or at 254nm for Diltiazem. A mixture of 0.1% triethylamine (pH3.0) and acetonitrile 10:1 for theophylline and 7:3 for Diltiazem were used as mobile phase at a flow rate of 1.0 mL/min. A linear detector response was observed over the concentration range of interest. In order to determine the drug release behavior of EC-coated granules contained 100 mg of drug, as a reference, JP dissolution test (Paddle method) was carried out, and the released amount of drug was determined by a UV spectrophotometer (UV-160, SHIMADZU Co., Ltd., Kyoto, Japan).
Theophylline and Diltiazem hydrochloride were selected as model drugs, and dissolution profile from controlled-release granules (containing 100 mg of drug) coated by porous EC membrane was evaluated. The results of the dissolution studies for controlled-release granules performed by the usual JP paddle method are shown in Figure 1. Figure 2 shows the results of dissolution studies from single granule in the controlled-release granules with porous EC membranes containing Theophylline or Diltiazem.
As shown in Figure 1, the dissolution profile of controlled-release granules containing Theophylline or Diltiazem showed almost similar trends with 3% porous EC coating for Theophylline and 6% porous EC coating for Diltiazem hydrochloride. The reason for the different coating rates for different drugs is that Theophylline has a solubility of less than 1% and Diltiazem hydrochloride has a solubility of more than 50%. The higher the solubility, the more polymer is required for dissolution control. From Figure 2, on the other hand, the dissolution profile for single granule is clearly different from a transition for granules by the JP paddle method, indicating that this is not due to the difference in coating amount. In the case of Theophylline, the transition to almost zero-order release occurred after the start of the dissolution test, whereas in the case of Diltiazem, the transition to zero-order release occurred after a lag time of several hours. In addition, Theophylline elutes almost linearly until the end of drug release, whereas in the case of Diltiazem, the rate of drug release decreases toward the latter half of the release period.
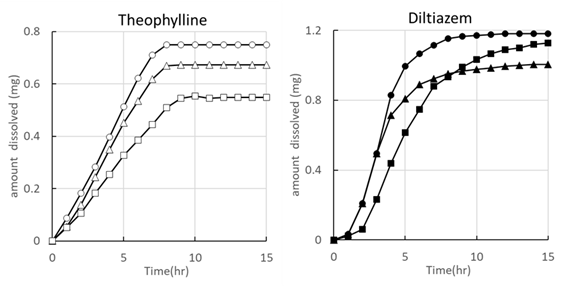
Figure 2 Each dissolution profile of theophylline and diltiazem release from single porosity-controlled ethylcellulose film coated granule (water 10ml).
These characteristics are summarized in Table 1. Short lag time of Theophylline is likely due in large part to its low film coverage, but it may also be due to the drug release mechanism from the porous EC film shown in Figure 3. The release mechanism by the porous EC film appears to be caused by a combination of three mechanisms: (1) penetration of the eluent for dissolution of the main drug component inside the granules, (2) zero-order release using osmotic pressure inside the granules after reaching saturation solubility, and (3) decrease in the release rate due to a decrease in osmotic pressure inside the granules caused by drug release. In the case of Theophylline, the lag time is shorter because of the low solubility, which allows less eluent to penetrate inside the granule, whereas in the case of Diltiazem, the solubility is higher (>50%), which allows more eluent to penetrate inside the granule. On the other hand, in the late phase of release, there is a decrease in the release rate due to a decrease in osmotic pressure.
These results taken together suggest that, even if the results of the dissolution test for granules are almost identical, it would seems to be worth to compare the detailed mechanism of drug release profile of single granule. Though not all granules were evaluated at this time, we intend to do so more accurately by establishing an automated system and a method to measure the amount of elution quickly.
None.
The authors declare that there are no conflicts of interest.
None.

©2024 Hiroyuki, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.