Journal of
eISSN: 2473-0831


Research Article Volume 9 Issue 1
1Department of Pharmaco-toxicology and Pharmacokinetics, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon
2Department of Pharmacognosy and therapeutic Chemistry, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon
3Department of Biochemistry, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon
4Department of Chemistry, Faculty of Science, University of Buena, Cameroon
5Department of Bio Resources, college of technology, the University of Bamenda, Cameroon
Correspondence: Charles Ntungwen fokunang, Department of Pharmaco-toxicology and pharmaceutics, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
Received: January 20, 2020 | Published: February 27, 2020
Citation: pougoue JK, Fokunang ET, Joseph EB, et al. Evaluation of antioxidant properties of secondary metabolites in aqueous extracts of Ficus: thonningii Blume tested on Wistar rats. J Anal Pharm Res. 2020;9(1):27?35. DOI: 10.15406/japlr.2020.09.00348
Background: Peptic ulcer is a major public health problem that affects about 10% of world’s population. It is a disease resulting from an imbalance between gastro protective and aggressive factors of the gastric mucosa. The treatment of this disease is usually long, expensive and also the difficulty in accessibility of the modern medications to the local population in rural zones. Peptic ulcer represent nearly 31.65% of cases of consultation in the services of gastroenterology in Cameroon. This makes poor patients rely on traditional medicine for their health problems. The objective of this study was to evaluate the antioxidant properties of secondary metabolites in aqueous extracts of Ficus. thonningii Blume tested on Wistar rats.
Material and methods: MDA, Catalase, Glutathione, Pepsin, SOD, ASAT, ALAT, Creatinine, XO, total proteins, were quantified using standard techniques. Phytochemical screening of plant aqueous extract for secondary metabolites was done.
Results: The extraction yield of the hydro-ethanolic extract (50:50) of the bark of F. thonningii Blume was 17%.
Phytochemical screening:The hydro-ethanolic extract had the greatest number of secondary metabolites at the end of the phytochemical screening. However, decoction and infusion extraction produced high level of polyphenol than the other extraction methods. Saponins was produced mainly from ethanolic maceration. The extract also showed a non-significant increase of SOD, Catalase and glutathione in the preventive and curative activity. A dose-dependent significant decrease p<.05 in MDA p<0,05 in the preventive evaluation.
Conclusion: This study showed that the hydro-ethanolic extract of the mixture of the dried bark of Ficus thonningii Blume had a promising antioxidant activity at 500 mg/kg. The administration of this extract at concentration up to 2000 mg/kg could have a potential effect of vascular protection and hepatic protection.
Keywords: Ficus thonningii stem bark hydro-ethanolic extract, phytochemical screening, antioxidant, wistar rat
Treatment of this disease requires in most cases a combination of several molecules with specific mechanisms of action. This treatment has 4 goals: relieve pain, accelerate healing, prevent complications and reduce the frequency of relapses. But while effective, treatment using conventional medicines is not usually well attended by patients.1 The reasons included their high cost and low availability to a large majority of the population especially those living in rural areas. In many developing countries, the health infrastructure is poor and a large majority of the population, mainly rural, has no access to primary health care and modern medicines. These patients use the resources of traditional herbal medicine as an alternative treatment.
However, traditional herbal medicine is facing a number problems for its vulgarisation including lack of sufficient studies on therapeutic properties as well toxicity tests to provide sufficient guarantees for their rational use. The common wild plant, F. thonningii, is extensively used in African ethno medicine for treating a number of disease conditions which include diarrhoea, urinary tract infections, diabetes mellitus, gonorrhoea, respiratory infections, and mental illnesses. The leaves of F. thonningii contain various bioactive compounds which include alkaloids, terpenoids, flavonoids, tannins and active proteins, all of which contribute to its curative properties. In vitro and in vivo pharmacological studies revealed that F.thonningii possesses antimicrobial, anti diarrheal, anti helmintic, antioxidant, anti-inflammatory and analgesic properties. Scientific research has validated the ethno medicinal claims that F. thonningii is useful in disease management. However, there is need to continue identifying, isolating and quantifying the active principles and possibly determine the mechanisms underlying the curative properties of its bark.2 It is in this context that the current study was conducted to investigate phyto chemically screen, evaluate the antioxidant of the stem bark extract on Wistar rats
This was an experimental in vitro and in vivo preclinical study on Wistar rats conducted from the 11 November 2016 to the 25th May 2017. The study was done in the Preclinical Animal toxicology and Pharmacology Laboratory of the Faculty of Medicine and Biological Sciences, of the University of Yaoundé 1, Cameroon, while the quantification of biochemical parameters was done in the biochemistry laboratory of the same university.
Ethical approval was given by the institutional review board (IRB) of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé 1 and administrative authorization was obtained to conduct study in the animal house of this faculty.
Fresh stem barks were harvested after identification by a botanist from the plant growing at Bafoussam on the 03rd of January 2017. The identified plant was authenticated at the National Herbarium of Cameroon by comparison with a sample having the voucher reference number 444042/HNC. The barks were dried under shade at room temperature for a period of three weeks in order to avoid solar radiations from altering the API. These barks were spread on plastic bags while avoiding their stacking. Every day we turned these barks upside down so as to favour a homogenous drying process. The dried barks were ground in a clean electric grinding machine in such a way to obtain a fined powder which was stored in an airtight container.
Three types of extraction procedures were used in order to evaluate the in vivo activity and selected the extract with the best activity since there were no studies with respect the evaluation of the antiulcer activity of the bark of Ficus thonningii Blume. These methods of extraction were:
In this process, the coarsely powdered crude plant was placed in a stoppard container with the solvent (distilled water, ethanol and hydro-ethanolic solution 50:50) and allowed to stand at room temperature for a period of 48 hours with frequent agitation until the soluble matter has dissolved. The mixture was then strained, the marc (the damp solid material) was pressed, and the combined liquids were clarified by filtration using Whatman paper.3
The yield (%) was gotten from the formula below:
For animal selection, the animals were subjected to a gross observation to ensure that the selected rats were in a good health. Rats were randomly selected with respect to body weight for final allotment to the study. The animal environment was made up of natural air conditioned rooms with optimal air changes per hour, relative humidity, temperature and illumination cycles set to 12h light and 12hours dark. The animals were accommodated in groups housed in cages with stainless steel grill top, together with facilities for food and water bottle and bedding of clean paddy husk.
For administration of the test substance, the plant extract was administered by oral gavage to each rat with 1ml of the ulcerogenic substance, using an intubation needle fitted onto a syringe of appropriate size. The dose administered to individual rat was calculated according to its body weight recorded on the day of test substance administration. The anti-ulcer reference drug used was omeprazole (OMIZEC) 20mg batch number 260044 bought in a community pharmacy in Yaounde specifically on the 02 January 2017.
This was done using the Mayer Waltz test, Hager Test and the Wagner test respectively.5
Tests was done using the Iron per chloride test, lead acetate test.4 In a test tube was introduced 2ml of the 1% extract and added a few drops of acetate of lead to 10 %. The formation of a white precipitate indicated the presence of polyphenols.
Use of Sodium hydroxide test: Sulfuric acid test.6 In a test tube 2ml of the 1% extract was put and added a few drops of Sulfuric acid then allowing them to flow over the tube wall. The formation of an orange coloration indicated the presence of flavonoids.
To 5ml of 5% extract was added 5ml of 10% H2SO4 followed by 5ml of ammonium hydroxide (NH4OH) diluted to half. In the presence of anthocyanin, the coloring was accentuated by acidification which turned into blue-purplish in basic medium.7
Test for identification of tannin (FeCl3 Test): In a test tube was introduced 5ml of the 5% infused in which was added 1ml of dilute aqueous solution of 1% ferric perchloride (FeCl3). The presence of tannins was indicated by blackish-blue or greenish coloration.8
Differentiation of catechic and gallic tannins: It was obtained by the reaction of stiasny, which was carried out in the following manner. To 30ml of infused solution was added 15ml of STIASNY reagent (10ml of 40% formalin more 5ml of concentrated HCl) and heated for 15minutes in a water bath at 90°C. With catechic tannins a precipitate showed their presence and for gallic tannins after filtration the filtrate was saturated with powdered sodium acetate, then 1ml of a solution of 1% ferric perchloride (FeCl3). The presence of gallic tannins was not precipitated by the Stiasny reagent was indicated by the development of dark blue shade.9
Mucilage Identification Test: To 1ml of decoctate at 10% was added 5ml of absolute ethanol. The obtaining of a fluffy precipitate indicated the presence of mucilages.10
The Foam Test: 100ml of the decoction at 1% were distributed in 10 test tubes numbered from 1 to 10 Successively 1, 2,10ml. The volume of each tube was adjusted to 10ml with distilled water. Each tube was stirred for 15seconds in the length direction and then left to settled for 15minutes and was observed for a persistence of the foam.11
Steroid Identification Test: In 1ml of extract was added 2ml of acetic anhydride then 2ml of sulfuric acid obtaining a violet colour. This colour turned blue or green to indicate the presence of steroids.11
Test for identification of resins: In a test tube was added 2ml of the 1% extract then a few drops of anhydrous acetic acid solution followed by 1ml of sulfuric acid (H2SO4) The appearance of a yellow colour indicated the presence of resins.12
Test for identification of cardiac glycosides (Keller-Killani test)13: In 0.5ml of the extract was added 2ml of glacial acetic acid and a few drops of 5% Ferric Chloride (FeCl3) solution, then 1ml of concentrated sulfuric acid. The Formation of a greenish or brown ring at the interface indicated the presence of glycosides.
Test for identification of quinones: In a test tube, put 2ml of 1% extract. Add 2ml of concentrated H2SO4. Obtaining a red colour indicated the presence of the quinones.14
In a test tube was put 2ml of the 1% extract then added 2ml of 2N NaOH and the tube was heated in a boiling water bath for 5minutes. The appearance of a yellow coloration indicated the presence of beta-cyane.15
In a test tube containing 1ml of the plant extract 1ml of distilled water was added with a few drops of 10% FeCl3. Obtaining a green or blue coloration that later turned yellow by addition of nitric acid (HNO3) indicated the presence of coumarins.16
In a test tube, was put 2ml of the 1% extract then added a few drops of ethanolic acid to obtain a greenish-black colour which indicated the presence of oxalates.17
Pepsin was used as a bio marker for the integrity of the gastric mucosa. In an acid medium, it hydrolyzed the peptide bonds of the proteins which contained the aromatic amino acids to give the polypeptides which, in the presence of the Folin reagent, gave a violet blue complex which exhibited a maximum absorption at 660nm. The intensity of the staining was proportional to the amount of polypeptide present in the solution.
Catalase19
The catalase assay was performed according to the method described by Sinha. 0.9ml of phosphate buffer(0.01M, pH 7) and 0.4ml of H2O2 were introduced into each tube to initiate the reaction. The reaction was interrupted after 30seconds by the introduction of 2ml of dichronic acetic acid. The whole was heated at 100°C for 10minutes. After cooling, the optical density was read at 570nm. The amount of hydrogen peroxide remaining in the solution after addition of the perchloric acid was evaluated using the calibration curve. The specific activity of catalase was expressed in μM H2O2 /min/mg protein.
Glutathione20
Reduced glutathione assay was performed according to the method described by Ellman (1959). The 2,2-dithio-5,5'-dibenzoic acid (DTNB) reacted with the SH groups of the glutathione to form a yellow coloured complex which absorbed at 412nm.
Malondialdehyde (MDA)21
The method used for the MDA assay was that of Wilbur et al.,21 Carbonyl compounds such as malondialdehyde from the decomposition of fatty acid hydro peroxides react with thiobarbituric acid (TBA) to give pink chromophores whose concentration was determined by reading the absorbance at 532nm.
Superoxide dismutase (SOD)22
Principle according to Mishra and Fridovich.,22 Adrenaline (epinephrine), in the presence of the superoxide anion O2, was oxidized spontaneously to adrenochrome; A colored compound which absorbed at 490nm. SODs, whose role was to reduce the O2 anion, inhibited this reaction
Expression of results:
The specific activity of SOD was evaluated in units of SOD/mg of protein. A unit of SOD was defined as the amount of SOD required to cause an inhibition of 50% of the oxidation of adrenaline to adrenochrome for one minute.
Proteins23
The quantification of proteins was done following the procedure described by lowry. This was described in the table below. The tubes were incubated for a period of 30 minutes at room temperature under shade, then read the optical density at 600 nm against the blank.
Xanthine oxidase25
The quantification of xanthine oxidase was done following the procedure described by Rousso et al..,25
The results were expressed in terms of mean±standard deviation. The comparisons between the groups were analyzed using one-way analysis of variance, the ANOVA test followed by Turkey's Kramer post hoc test using the GraphPad Instat version 5.0 software. A P-value of less than 0.05 was considered statistically significant.
The extraction yield of the hydro-ethanolic extract (50:50) of the bark of F. thonningii Blume was 17% that was selected for use in the study.
The hydro-ethanolic extract had the greatest number of secondary metabolites at the end of the phytochemical screening as shown in table 1. However, decoction and infusion extraction produced high level of polyphenol than the other extraction methods. Saponins were produced mainly from ethanolic maceration (table 2).
Put in the test tubes |
Sample |
white |
Sodium hydroxide 0.1N |
190µL |
200µL |
Gastric juice |
10µL |
|
Solution C |
1000µL |
1000µL |
Incubate for 10 minutes |
||
Solution D |
100µL |
100µL |
The tubes were then vortexed |
Table 1 Description of the protein quantification
Test |
Specific test |
DEC |
INF |
ET MAC |
H/E MAC |
AQ MAC |
Polyphenols |
FeCl3 |
˖˖˖˖˖ |
˖˖˖˖˖ |
˖ |
˖˖ |
˖ |
lead acetate |
˖˖˖ |
˖˖ |
˖ |
˖ |
_ |
|
Saponin |
˖ |
˖˖ |
˖˖˖ |
˖˖ |
_ |
|
Mucilage |
_ |
_ |
_ |
_ |
_ |
|
Alkaloids |
WAGNER |
_ |
_ |
˖ |
˖ |
_ |
MAYER |
_ |
_ |
˖ |
˖ |
_ |
|
HAGER |
_ |
_ |
˖ |
˖ |
_ |
|
Flavonoids |
NaOH |
˖ |
˖ |
˖ |
˖ |
_ |
H2SO4 |
˖ |
˖ |
˖ |
˖ |
˖ |
|
Tannins |
CU2SO4/NH3 |
˖ |
˖ |
˖ |
˖ |
_ |
catechic |
˖ |
˖ |
˖ |
˖ |
_ |
|
gallic |
_ |
_ |
_ |
_ |
_ |
|
Steroids |
acetic anhydride |
_ |
_ |
_ |
_ |
_ |
Coumarines |
FeCl3 |
˖ |
˖ |
_ |
_ |
_ |
HNO3 |
˖ |
˖ |
˖ |
˖ |
_ |
|
Oxalate |
_ |
_ |
_ |
_ |
_ |
|
Quinones |
˖ |
˖ |
_ |
˖ |
˖ |
|
beta cyanide |
_ |
_ |
_ |
_ |
_ |
|
Phlobo tannins |
˖ |
˖ |
˖ |
˖ |
_ |
|
Antho cyanide |
˖ |
˖ |
˖ |
˖ |
_ |
|
Cardiac glycosides |
_ |
_ |
_ |
_ |
_ |
|
Resins |
˖ |
˖ |
_ |
_ |
_ |
Table 2 Presentation of the secondary metabolites in the aqueous extract of the bark of F. thonningii Blume
DEC- Decoction; INF- Infusion; ETMAC-Ethanolic maceration/E-Hydroethanolic maceration; AQ- Aqueous maceration; – represents the absence of metabolites, + represents the presence of metabolites, ++ abundant and +++ very abundant, ++++ extremely abundant
For catalyse quantification, there was a gradual increase of the catalyse concentration across the negative control group to the healthy animal group (Figure 1). MDA quantification showed a significant dose-dependent decrease in MDA in the groups receiving the extract as compared to the negative control group, though it was highest in the 500mg/Kg group. The MDA content was lowest in healthy groups than omeprazole (Figure 2)
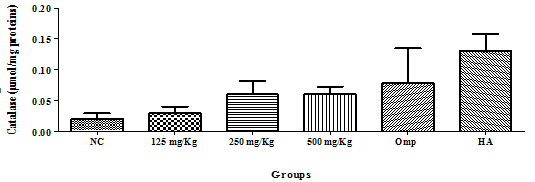
Figure 1 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of catalase (P<0.05). p. NC=Negative control; Omp=Omeprazole, healthy animal.

Figure 2 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of MDA (P<0.05).
Glutathione: There was no significant variation in the glutathione level, but amongst the test groups, the 500mg/Kg group has the highest level as compared to the negative control group (Figure 3).
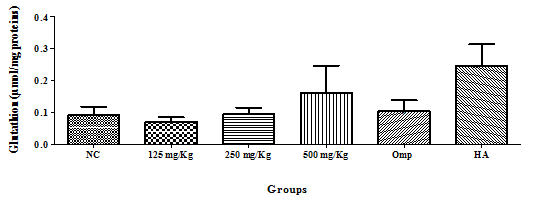
Figure 3 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of glutathione (P<0.05).
Sulfoxide dismutase (SOD): There was no significant variation in the glutathione level, but the healthy animal (HA) group had the highest level as compared to the negative control group (figure 4). Variation of pepsin concentration, mucus concentration and gastric juice.
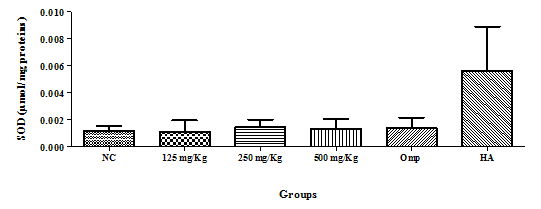
Figure 4 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of SOD (P<0.05).
There were no significant changes at all the extract concentration for pepsin and mucus concentration compared to the positive control omeprazole. However, a significant difference (p<0.05) was observed across the dose range when compared to the positive control (Table 3). There was a great reduction of gastric juice 3,00±1,66 at 500mg/Kg compared with 5,15±1,44 recorded for omeprazole (Table 3).
Parameters |
NC |
125mg/Kg |
250mg/Kg |
500mg/Kg |
Omp |
HA |
Pepsine(µmol/mg pr) |
0,07±0,02 |
0,03±0,02 |
0,03±0,01 |
0,04±0,04 |
0,05±0,02 |
0,03±0,02 |
Mucus (mg/mg pr) |
0,46±0,13 |
0,41±0,08 |
0,45±0,07 |
0,42±0,08 |
0,03±0,02*** |
0,02±0,05*** |
Gastric juice (ml) |
5,55±1,40 |
4,33±1,59 |
4,50±2,47 |
3,00±1,66 |
5,1±1,44 |
0,25±0,00*** |
Table 3 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentrations of pepsin and mucus, as well as the gastric juice variation (P<0.05)
Pr- proteins, NC-negative control, HA- healthy animals, Omp- omeprazole
Glutathione: There was no significant variation in the glutathione level, but amongst the test groups, the 500mg/Kg group has the highest level as compared to the negative control group (Figure 5).
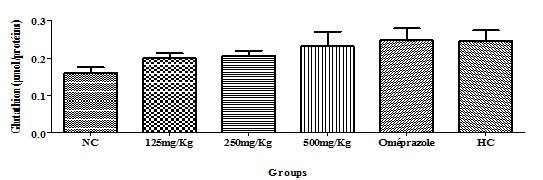
Figure 5 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of glutathione (P<0.05).
Catalyse: There was a dose dependent increase in catalyse activity with increasing dose from 125mg/kg body weight to 500mg/kg body weight (Figure 6) At high dose of extract 500mg/kg there was increased catalyse activity compared to the reference drug omeprazole. There is a gradual increase of the catalyse concentration as we move from the negative control group to the 500mg/Kg group (Figure 6)
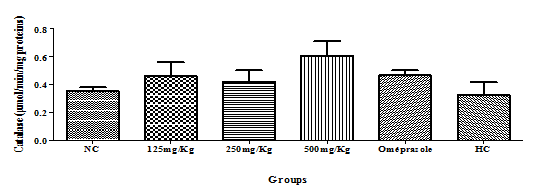
Figure 6 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of catalase (P<0.05).
Malondialdehyde (MDA): There was a significant difference in the decrease in MDA activity compared to the negative control (Figure 7). However, the decrease in MDA among the extract dose ranges were not significant compared with the reference drug omeprazole, but significant difference could be seen when compare to healthy animal treatments (Figure 7). There was very little MDA. We observed a non-significant dose-dependent decrease in MDA in the groups receiving the extract as compared to the NC, though it was high in the 500mg/Kg group. The MDA content was lowest in healthy groups (Figure 7).
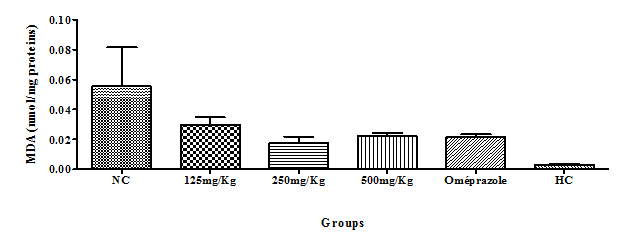
Figure 7 Effect of the hydro-ethanolic extract of F. thonningii Blume on the concentration of MDA (P<0.05).
Xanthine oxidase: There was a non-significant dose-dependent decrease in Xanthine oxidase in the groups receiving the extract as compared to the control treatments. However, the decrease was significant from 250mg/kg-500mg/kg that was comparable to omeprazole (Figure 8).
Belonging to the family of Moraceae, the genus Ficus is among the largest genera of angiosperms, from about 60 are present in Cameroun.3,12,26 In our search of bioactive compounds from Cameroonian medicinal plants of the Ficus, we examined the hydro-ethanolic extract of the bark of F. thonningii Blume on peptic ulcers induced by absolute ethanol. Ethanol exposes the mucosa to the proteolytic and hydrolytic actions of hydrochloric acid and pepsin,7,28 causing damage to the membrane,13 stimulates acid secretion, increases activity of xanthine oxidase, triggers imbalances in cellular antioxidant processes, reduces mucosa microcirculation and increases apoptosis.16,29
In this study, the results of the phytochemical screening showed that the hydro-ethanolic stem bark of F. thonningii Blume contained various biologically active compounds called phytochemicals, which are naturally produced by the plant as protection against biotic and abiotic stresses. The main groups of phytochemicals isolated from the prepared extract solution included; polyphenols, saponins, alkaloids, flavonoids, catechic tannins, coumarins, quinones, phlobotanins, anthocyanins which corroborates with the work done by Dangarembizi. in 2013 on the leaves of F. Thonningii,10,30 and Usman et al.5 in 2010.31 These metabolites are similar to those found in F. Sycomorus;7,32 Phytochemicals such as alkaloids have anti-depressive and anti-inflammatory effects in which some have preventive and curative anti-ulcer activities,19,33–34 flavonoids favours blood circulation, are antioxidants as well increase the production of prostaglandins in the gastric mucosa.16,352 Most of these phytochemicals have an effect on the gastric mucosa which could be responsible of the plant antiulcer activity.
Benjamin, who showed that the mixture Tetrapleura tetraptera and Guibourti aehie inhibited to 91.5% the ulcers induced by the HCl/ethanol mixture at 500mg/kg.4,36–37 Ethanol also reduces the activity of antioxidant enzymes such as SOD, catalase and glutathione. It significantly increases the amount of MDA than that the healthy control. The role of these enzymes in the defense of the organism against oxidative stress is well known. SOD, for example, provides the first line of defense by converting superoxide free radicals to hydrogen peroxide. The latter is then degraded by catalyse in water.9,38 As for glutathione, it interacts directly with active oxygen species and ensures the elimination of peroxidized lipids such as MDA.34,39 Thus, the increase in the level of these enzymes (glutathione, SOD. catalyse) in animals that received F. thonningii hydro-ethanolic stem bark extract and omeprazole show that the inhibition of gastric ulcers in these gastric ulcers is also due to an antioxidant activity. Jing-Yang Wong et al. in 2013 did a similar study in which the aqueous extract of H. Erinaceus significantly decreased lipid peroxidation and resulted in increased SOD and catalyse activity, indicating its efficacy in the Prevention of gastric ulcers induced by ethanol in rats.13 thonningii Blume stem bark hydro-ethanolic extract at all doses (125,250 and 500mg/Kg) have reduced the ulcer total surface area when compared to the negative control group (P<0.05) where the greatest percentage of inhibition was observed by the 500mg/Kg group of rats (92.94%) but not significantly. This results did not corroborate with the Curative treatment of detoxified pericarp extract of Anamirta cocculus fruit given orally at a dose of (200mg/kg) and roxatidine (positive control) administered at a dose of (100mg/kg) which induced a significant curative effect. The anti-ulcer drug, roxatidine and detoxified pericarp extract of Anamirta cocculus fruit significantly inhibited ulcer formation by 81.06% and 54.49% respectively when compared with control (p<0.001).32,40–41 The present finding suggests that F. thonningii Blume stem bark hydro-ethanolic extract promote ulcer protection as ascertained by the comparative decrease in ulcer surface area and percentage inhibition of ulcers. In the present alcohol-induced gastric ulcer model, the levels of glutathione, catalase and MDA increased in the 500mg/Kg group as compared to the negative control. These compounds are important for maintaining the integrity of the gastric mucosa and mediating the protective effects of prostaglandins against gastric mucosal injury.41
About one hundred proteins are present in the plasma, they are albumin. Most are made by the liver. They are involved in transport of different substances in the blood including lipids (fatty acids), iron or many drugs. They also participate in blood coagulation, immune defense maintaining blood pressure.15,21 Their dosage is used to evaluate the state of hydration, nutritional status, liver, kidney function, or other disease states Inflammation or impaired immune defenses. A reduction in protein is observed in cases of intestinal malabsorption, malnutrition, liver disease, immune deficiency, exocrine pancreatic insufficiency, kidney disease, severe exudative dermatitis, extreme hemorrhage, enteropathies exudative, and infiltrations of the intestine, while an increase in proteins is observed in cases of dehydration, chronic inflammatory process, globulin abnormalities (lymphoma, plasmacytoma), intravascular haemolysis, hyper bilirubinemia and of lipemia.4,24,37
We can conclude from this work that the F.thonningii Blume stem bark hydro-ethanolic extract- contains flavonoids, saponins, quinones, alkaloids, coumarins, catechic tannins, polyphenols, flavonoids, phlebotanins and Antho cyanides. At 500mg/kg, this extract inhibited pepsin concentration, XO and MDA; increased, glutathione, catalase activity and SOD. This extract showed a promising pharmacologic and therapeutic activity for the management of ulcers by a gastro protective, anti-ulcer and antioxidant action and not by an antacid action. Administered at 2000mg/kg, would have a vascular protective effect. This study has also proven the use of Ficus thonnningii in category 1 phyto medicne when use as extemporaneous preparation by tradi practioners, and therefore the possibility of further study for a galenic formulation as an improved traditional product.
None.
The author declares that there are no conflicts of interest.
None.

©2020 pougoue, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.