Journal of
eISSN: 2473-0831


Research Article Volume 8 Issue 1
Department of Pharmacy, Southeast University, Bangladesh
Correspondence: Md. Shariful Islam, Department of Pharmacy, Southeast University, Banani, Dhaka-1213, Bangladesh
Received: December 11, 2018 | Published: February 28, 2019
Citation: Munira M, Islam S, Akther N, et al. Estimation of anti-inflammatory, analgesic and thrombolytic activities of Sonneratia caseolaris Linn. (Family: Sonneratiaceae). J Anal Pharm Res. 2019;8(1):20?23. DOI: 10.15406/japlr.2019.08.00305
In this study, Sonneratia caseolaris was evaluated for its anti-inflammatory, analgesic andthrombolytic activities using different systems assay. The anti-inflammatory activity,the % of inhibition of paw edema of crude methanol extract showed the highest activity in leaf (58.24% inhibition) at 100µg/ml, in bark (40.80% inhibition) at 200µg/ml.The analgesic activity, the effect of each extractive at two different concentration (100 & 200µg/ml) on the response of mice was measured. The most potent activity was also found in crude methanolic fraction (CME) showed in leaf highest % of inhibition (25.93 at 100µg/ml) and in bark highest % of inhibition (33.33 at 100µg/ml) after standard (Diclofenac-Na). Hence, it is worthwhile to isolate and elucidate the bioactive principles that are responsible for the better activity that is underway.
Keywords: anti-inflammatory activity, thrombolytic activity, analgesic activity, Sonneratia caseolaris
The variety of medicinal plants is increasing around the world includes more than a thousand species.1 In Bangladesh more than 500 of these medicinal plants have been reported right now. Medicinal plants have long been extensively used in preparation of ayurvedic, unani, homeopathic medicines in our country and the proper scientific evaluation of the species limits their uses in therapeutic applications. S. caseolaris is a mangrove1 species belonging to family Sonneratiaceae.2 S. caseolaris is a widespread and can be found in Bangladesh, China, Indonesia, India, Malaysia, Philippines, Myanmar, Singapore, Sri Lanka, Thailand, Northeast Australia, Cambodia, Maldives, Solomon Islands and New Caledonia. S. caseolaris is a medium is medium-size plant (2-20m height), evergreen tree with elliptic-oblong leaves (5-9.5cm long).3,4. Flowers solitary at the end of braches about 5cm across and fruits are a fleshy berry supported by persistent calyx, depressed globose (5-8cm across). S. caseolaris have 24 compounds such as nine triterpenoids, eight steroids, three flavonoids and four benzene carboxylic derivatives have been isolated from stems and twigs of medicinal mangrove plant S. caseolaris.5 This plant contains phenolic compound like gallic acid and flavonoids e.g. luteolin and luteolin-7-O-glucoside.6 S. caseolaris to be used in traditional medicine systems in several countries, it is used for sprains, swelling helminthiasis, poultices, coughs, hematuria, small pox, astringent, antiseptic, arresting hemorrhage, piles, and also used as remedy to stop blood bleeding.7 S. caseolaris possessed intestinal α-glucosidase inhibitory property9 and it has also been reported to be toxic against mosquito larvae.7 The present study was started to identified the pharmacological parameter of extracts from mature leaves and barks of S. caseolaris because that it may contains substances with potential therapeutic uses and these could serve a basis of precursors for synthesis of useful drugs.
Plant material
The leaves of S. caseolaris were collected from Barisal District, Bangladesh, in April 2014, and identified by an expert taxonomist. A voucher specimen was submitted to the national herbarium, Mirpur, Dhaka, Bangladesh.
Preparation of the extract
Powdered plant materials (leaves) having a weight of about 340gm were taken in an amber colored reagent bottle and soaked in 1.5 liter of methanol and barks having a weight of 310gm were taken in an amber colored reagent bottle and soaked in 1.5 liter of methanol for about 14 days with at room temperature with occasional shaking. The whole mixtures was filtered using cotton filter and then through Whitman’s No.1 filters paper and was concentrated with a rotary evaporator under reduced pressure at 55°C temperature to afford crude extract (12.995gm).
Animals
A total number of 48 young Swiss-albino mice (20-30gm) of either sex were collected from Animal Research Branch of the International Centre for Diarrhoel Disease and Research Bangladesh (ICDDR’B), Mohakhali, Dhaka. They were kept in standard laboratory conditions for 1 week for acclimation after their purchase and feed ICDDRB formulated rodent food and water.The experiments were done in an isolated and noiseless room.
Drugs and chemicals
Indomethacin, Diclofenac Na, (Square Pharmaceuticals Ltd.) Carrageenan (Human, Germany), 0.9% NaC1 (Beximeo Infusion Ltd.),DPPH (Sigma chemical company, USA), Methanol (Sigma chemical company, USA), Butylated hydroxy toluene (BHT) (Merck, Germany), Vincristine sulphate (Sigma chemical company, USA),Acetic acid (Merck, Germany), and Other chemicals were obtained from local sources and were of analytical grade.
Analgesic activity
In this method, Koster et al.,8 acetic acid is administered intra peritoneally to the experimental animals to create pain sensation. As a result, the animals squirms their body at regular interval out of pain. This squirm or contraction of the body is termed as “writhing”. As long as the animals feel pain, they continue to give writhing. Each writhing is counted and taken as an indication of pain sensation. Any substance that has got analgesic activity is supposed to lessen the number of writhing of animals within in a given time frame and with respect to the control group. The writhing inhibition of positive control was taken as standard and compared with test samples and control. As positive control, any standard NSAID drug can be used. In the present study, Diclofenac-Na was used to serve the purpose.
Anti- inflammatory activity
In the present study, anti-inflammatory activity described by Winter et al.,9 the possible anti-inflammatory actions of the extracts were investigated in comparison with the indomethacin administered experimental animals. The anti-inflammatory action was evaluated by using carageenan-paw induced edema. Measurement of paw volume was done by means of volume displacement technique using .Thin thread immediately after carrageenan injection and after 1, 2, 3, and 4hr.
% of inhibition were obtained using the following ratio:
(Vt − Vo) control − (Vt − Vo) treated
(Vt − Vo) control × 100. (1)
Where, Vt is the average volume for each group after treatment and Vo is the average volume for each group before any treatment.
Thrombolytic activity
In vitro clot lysis activity of the leaves of S. caseolaris was carried out according to the method of Prasad et al. [10],with minor modification.Venous blood drawn from healthy volunteers (n=13) was immediately citrated using 3.1% sodium citrate solution and then was transferred in different pre-weighed sterile micro-centrifuge tube (500μl/tube). Two hundred microlitres of 2% calcium chloride was then added to each of these tubes, mixed well and incubated at 37°C for 45 minutes for clotting to occur.Briefly, after clot formation, serum was aspirated out without disturbing the clot formed and each tubehaving clot was again weighed to determine the clot weight (Clot weight=weight of clot containing tube –weight of tube alone). Each micro centrifuge tube containing clot was properly labeled and five hundred microlitres of different concentrations of the plant extract, 2.5mg/ml (n=13), 5 mg/ml (n=13), 10 mg/ml (n=13) and 20 mg/ml (n=13) or saline (negative control) (n=13) or 30,000I.U. of streptokinase [(Eptase, the Beacon Pharmaceuticals Ltd, Bangladesh), reference drug (n=13)] was added to tubes with clots. All the tubes were incubated at 37oC for 90 min. The fluid left was then carefully removed and the tubes were weighed again. The difference in weight before and after clot lysis was expressed as % clot lysis. Streptokinase and water were used as a positive and negative control respectively. All experimental procedure was repeated for three times.
% Clot lysis=(Weight of the lysis clot / Weight of clot before lysis) × 100
Analgesic activity
Diclofenac-Na like non-narcotic analgesic activity of the test extracts was investigated by the ability to protect a painful writhing syndrome in mice. The Writhing was consistently produced in mouse by an intraperitoneal injection of 0.7% aqueous acetic acid. Overnight fasted, healthy adult male albino Swiss mice weighing between 25 to 30gm in groups of four each were taken for present investigation. DMSO 1% solution of the test extracts were administered orally in a dose of 50mg/kg, 100mg/kg and 200mg/kg body weight respectively to the test groups animal. The control group of animals were given only DMSO 1% solution in the dose of 10ml/kg body weight. One group of animal was administered with Diclofenac sodium as standard, orally in a dose of 10mg/kg (b.w). After a gap of 30 minutes of the administration of the test extracts, all the groups of mice were given the writhing agent, 0.7% aqueous acetic acid, in a dose of 1ml/100gm (b.w) intraperitoneally. Five minutes after administration of acetic acid the number of writhing produced in these animals were counted for next 10 minutes and the number of writhing produce in the tested groups were compared with those in the control group and the percentage protection was calculated as show below (Table 1) (Figure 1 & Figure 2).
Groups |
Treatment |
Dose |
Avg. no. of writhing |
% Inhibition |
01 |
Control (1% DMSO) |
10ml/kg |
85 .00±1.707 |
- |
02 |
Diclofenac-Na |
10mg/kg |
3.75 ±0.957 |
95.58 |
03 |
leaf |
100mg/kg |
21.25±1.25 |
74.29 |
04 |
200mg/kg |
18.50±0.577 |
78.23 |
|
05 |
bark |
100mg/kg |
22.50±1.290 |
73.52 |
06 |
200mg/kg |
22.25± 1.500 |
73.82 |
Table 1 Evaluation of analgesic activity of extract of Sonneratia caseolaris by acetic acid induced writhing method
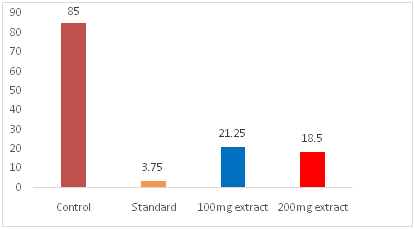
Figure 1 Effects leaf of Sonneratia caseolarison acetic acid-induced writhing in mice.
Among these fractions the most potent activity was also found in crud methanolic fraction (CMF) show highest % of inhibition(78.23 at 200µg/ml) after standard (Diclofenac-Na).
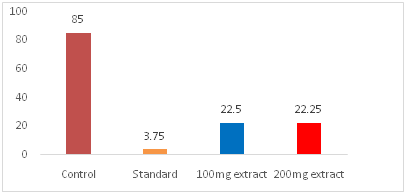
Figure 2 Effects bark of Sonneratia caseolarison acetic acid-induced writhing in mice.
Among these fractions the most potent activity was also found in crud methanolic fraction (CMF) showed the highest % of inhibition (73.82 at 200µg/ml) after standard (Diclofenac-Na).
Percentage protection=[(No. of writhes in control - No. of writhes in test) ÷ No. of writhes in control] x 100
Anti-inflammatory activity
The different test extracts were administrated to the animals in the test groups at the dose of 50, 100 and 200mg/kg by oral route. Animals in the standard group received Indomethacin at the dose of 10mg/kg, by oral route. Control group animals were received 1% DMSO at the dose of 10ml/kg body weight. Thirty minutes after administration of the respective drugs, all the animals were challenged with 0.1ml of 1% carrageenan in the sub planter region of left hind paw. Paw volume was measured by using digital plethysmometer before administration of carrageenan and after 30min, 1, 2, and 3 hrs intervals. The efficacy of different drug was tested on its ability to inhibit paw edema as compared to control group. The percentage inhibition of paw edema was calculated by the formula as below (Table 2) (Figure 3).
Treatment |
Dose |
Paw volume at different time interval (in mm) |
|||||||
1hr |
% inhibition |
2hr |
% inhibition |
3hr |
% inhibition |
4hr |
% inhibition |
||
Control |
Vehicle |
3.96±0.8 |
- |
5.16±0.70 |
- |
2.5±3.06 |
- |
5.46±1.55 |
- |
Standard |
10mg/kg |
0.3±0.2 |
92.43 |
0.52±0.3 |
89.92 |
1.42±2.56 |
94 |
0.275±0.17 > |
94.96 |
Methanolic extract |
100mg/kg |
2.66±0.56 |
32.83 |
2.96±1.93 |
42.64 |
1.88±0.54 |
56 |
2.28±0.79 |
58.24 |
|
200mg/kg |
2.58±0.23 |
34.85 |
3.2±0.96 |
37.98 |
1.86±0.35 |
27.38 |
2.16±0.08 |
60.43 |
Table 2 Determination of volume of edema of mice at different time for of Sonneratia caseolarisleaf
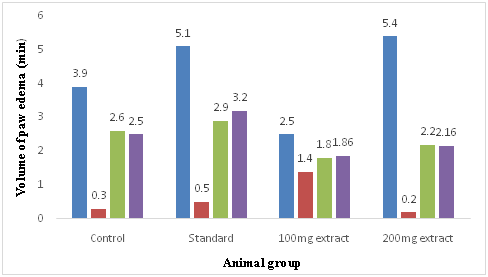
Figure 3 Graph of volume of edema of mice at different time for of Sonneratia caseolaris leaf.
The LD50 of the test samples were calculated using the concentration versus % of nauplii mortality curve of the samples. The lower LD50 means higher toxicity. Among all extracts of Sonneratia caseolaris methanol fraction showed the highest cytotoxicity activity with LD50 value of 14.58mg/ml.
% of Inhibition of Paw edema=[(VC - VT )/ VC ] × 100
Where, VC= Paw edema of control group and VT=Paw edema of treated group.
Thrombolytic activity
Clot lysis of blood samples of normal subjects by different concentrations of crude methanolic extracts leaf and bark of Sonneratia caseolaris. The clots were treated by four different concentrations of extracts such as 20, 10, 5 and 2.5 mg/ml and clot lysis % was 26.27& 44.67% of leaf and bark respectively (Table 3) (Figure 4).
Extract/ Drug |
% clot lysis |
Distilled water |
3.62 |
Streptokinase |
57.86 |
Methanolic extract of leaf of Sonneratia caseolaris |
26.27 |
Methanolic extract of bark of Sonneratia caseolaris |
44.67 |
Table 3 Effect of methanolic crude extracts whole plant of Sonneratia caseolaris on blood clot lysis of human blood in vitro
This study examined the thrombolytic potential of crude methanolic extract of leaf bark of Sonneratia caseolarisin vitro using human blood. The test model used is a newly developed cheap and simple technique which can be performed with limited facilities available in countries like Bangladesh. But this technique is validated, sensitive and reliable. The results show, for this methanolic extract of both drug possesses thrombolytic activity. This is an important finding which may have important implications in cardiovascular health. In addition, this finding may indicate the possibility of developing novel thrombolytic agents from the extract of the plant. Thrombolytic activity of the plant extract was rapid and showed a dose related trend (in terms of regression analysis) indicating that the effect is genuine and is selective. The methanolic crude extracts of leaf possess slightly significant thrombolytic property. It should be further investigated. The methanolic extract of bark show extremely significant peripheral analgesic property at both high and low doses. This property showed is investigated. It is evident from result section that Sonneratia caseolaris leaf has good anti-inflammatory activity.
From this study, it was concluded that the phytochemical screening of the methanolic extract of the leaf and bark of Sonneratia caseolaris contain some bioactive components and these pharmacological properties are attributed to their phytochemical constituents. So, further studies are in progress to isolate the active constituents responsible for the observed effect.
The authors are grateful to the Department of Pharmacy, Southeast University, Bangladesh, for their support during the research and also thankful to Bangladesh National Herbarium, Dhaka, Bangladesh for their identification.
The conducted research is not related to either human or animals use.
Authors have declared that no competing interests exist.

©2019 Munira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.