Journal of
eISSN: 2473-0831


Short Communication Volume 7 Issue 1
Correspondence: Soriano-Garc, Tel 5255-5622 4569, Fax 5255 5616 2217
Received: January 29, 2018 | Published: February 8, 2018
Citation: Soriano-García MM, Victoria MTC (2018) Electrostatics at the Active Site of Two ?-Amylases: Direct Comparison of Experiment with Theory. J Anal Pharm Res 7(1): 00201. DOI: 10.15406/japlr.2018.07.00201
The vital importance of electrostatics in catalysis has been emphasized in the literature for a large number of enzymes. The effect of pH on the rate of hydrolysis of both sorghum (Montecillo 2 and 3) hybrids starches by industrial bacterial amylase (IBA) and Aspergillus oryzae α-amylase (AOA) was studied. This result suggests that among the two amylases compared, both IBA and AOA α-amylases had slightly high affinity for soluble starch of sorghum Montecillo 3 and 1 hybrids, respectively. We examined the differences in the pH-activity profiles for the industrial bacterial amylase (IBA) and Aspergillus oryzae α-amylase (AOA) acting on both sorghum (Montecillo 2 and 3) hybrids starches which show a real change in the net charge on the active site. Both our experimental data and prediction studies indicated that the average pKa values are in good agreement with the predicted values of 5.44 and 6.59.
Keywords:aspergillus oryzae amylase, industrial bacterial amylase, electrostatics, pka’s, ph activity profile; sorghum starches
Electrostatic interactions are of vital importance in diverse aspects of protein structure and function,1–5 including the catalytic activity,6,7 ligand binding,8 complex formation,9\ proton transport,10,11 as well as their stability of folded proteins.12,13 These interactions involve full charges on the side chains of ionizable amino acids that arise from the association and dissociation of protons to and from these acid-base groups in the protein.
Electrostatics is well understood from the theoretical point of view, and several computational procedures for calculating the electrostatic field in macromolecules have been published. Programs for solving the Poisson-Boltzmann equation, for example DELPHI,14 GRASP15 and UHBD16 are well-known tools used in many laboratories around the world. The influence of electrostatics on catalysis is not well understood mainly due to the fact that theory and experiment are not in good agreement.17
Starch is a reserve chemical form of the energy of the sun found in green leaves, stems, cereal grains, seeds, nuts, and fruits of plants. Many kinds of starches provide the major source of food energy in the diets of humans and are increasingly used industrially as bio-renewable materials for the formation of a diverse array of products, e.g. ethanol, high-fructose syrups, cyclomaltodextrins, maltodextrins, adhesives, paper and textile sizing agents, and so forth.
Sorghum is an important cereal grain resistant to water drought and extreme high temperatures with more than 60 million metric tons harvested from 44 million hectares in 2015.18 Sorghum is a staple food in many areas of Africa (Nigeria, Sudan, Burkina Faso, Ethiopia), Asia (India, Middle East Pakistan and Northern China), and the drier parts of Central and South America (Argentina, Bolivia and Mexico),19Australia and the United States. Except for Australia and the United States, the rest of those areas the populations are frequently undernourished and it is important to consider sorghum starch and protein digestibility. It is estimated that more than 40% of the worldwide sorghum production is used for human food consumption.20
α-amylases[EC 3.2.1.1] are widely distributed enzymes formed by bacteria, fungi, plants and animals that hydrolyze the α-(1→4)-glucosidic bonds of starch into maltodextrins of varying sizes, depending on the particular enzyme.21 The vital importance of electrostatics in catalysis has been emphasized in the literature for a large number of enzymes. The effect of pH on the rate of hydrolysis of both sorghum (Montecillo 2 and 3) hybrids starches by industrial bacterial amylase (IBA) and Aspergillus oryzae α-amylase (AOA) was studied.
The electrostatic fields can be studied by measuring the characteristics that in part or fully determined by the electrostatic field in the protein, pKa values are known to be influenced by the electrostatic field.22 and in the case of some enzymes, the substrate binding properties and the pH dependence of catalysis have also been used to study changes in the electrostatic field.23 Knowledge of the protonation state of the residues at the active site is required for a thorough understanding of these electrostatically controlled phenomena in quantitative detail.
The pKa values of the ionizable side chains at the active sites in amylases from two different sources acting on two sorghum (Montecillo 2 and 3) hybrids starches are a function of the electrostatic properties of the surrounding microenvironment of the active site.
Materials
Sample preparation: Sorghum starches were freshly prepared from the two mature sources (Montecillo 2 and 3) hybrids. Mature sorghum seeds were obtained from Dirección del Instituto de Recursos Genéticos y Productivos del Colegio de Posgraduados in Montecillos, Estado de México, México. Industrial bacterial α-amylase was obtained from Gitss Brocades and Aspergillus oryzae α-amylase was a crystalline enzyme obtained from Sigma Chemical Co.
The starch extraction was performed using a modified procedure of Schoch et al.24 The sorghum seeds are cleaned and milled using a commercial mill. The sample was sieved through a 3mm mesh. This material was re-suspended in water with a ratio 1:3 (w/v) with 50 ml of toluene to avoid any microorganism. The pH of this mixture is maintained between 5.9 to 6.3, to avoid any acid starch hydrolysis. The starch suspension was filtered through a nylon mesh and adding cooled water at 5°C continuously until the filtered water is clear. This precipitate is re-suspended on distilled water with a ratio 1:3 and again filtered using a nylon mesh and refrigerated at 4°C during 16 h. Then the water is removed and the precipitated is re-suspended in a solution of sodium hydroxide 0.2% in a ratio 1:2 to eliminate the protein fine fiber in this precipitate. This procedure is followed twice and maintaining its pH at 6.0. All starch obtained is dried at room temperature.
The starch granules were solubilized by suspending 1g in a 0.02M phosphate buffer at pH 6.9 and 0.007M of NaCl, and maintained this suspension under boiling conditions for 3 or 4 min with continuous and vigorous stirring, followed the dilution with the same buffer solution to reach 100 ml.
Assays of the α-amylases: The activities of industrial bacterial (IBA) and Aspergillus oryzae α-amylases (AOA) were determined by the measurement of the increase in the reducing value of maltodextrin products of the reaction. The reducing value was determined by the Miller method,25 using maltose as standard. This method is based on the quantification of reducing groups formed by the action of α-amylase on the starch molecule.
For the assays, a 1% soluble starch was prepared by dissolving 1g of soluble starch in 0.02M phosphate buffer at pH 6.9 and 0.007M NaCl and then boiled this solution and rigorously stirred during three or four min, then cooled and calibrated to 100ml with the same buffer. In the assays, 1.9ml of the 1% soluble starch solution that were pre-incubated at 37°C for 10 min to reach the desirable temperature and then added 0.1ml of the enzymatic solution. At the end of this time, 4 mL of 3,5-dinitrosalycilic acid was added in order to stop the reaction, and vigorously stirred to homogenized the mixture. The formation of 3-amino, 5-nitrosalylic acid is carried under boiling water environment during five min, then cooled and read at 540 nm. Triplicate assays were made for both starches and enzymes. All buffer solutions used in all experiments at different pH values have an ionic strength of 0.1M.
Activities for IBA and AOA
The effect of pH on the rate of hydrolysis of both sorghum (Montecillo 2 and 3) hybrids starches by IBA and AOA have been reported.26 The curves shows an optimum pH’s for the fungal and bacterial amylases between 5.5-6.0 and 6.0-7.0, respectively. These values are in agreement with the optimum pH values found for amylase from germinated sorghum starch and amylase from the cover of germinated sorghum;27 malt amylase;28 amylase of B. licheniformes;29 amylase from B. subtilis30 with values of 6.5-7.0, 6.5-7.5, 5.0-6.0, 5.0-7.0 and 5.8-6.0, respectively.
α-Amylase (α-1,4-glucan-4-glucanohydrolase, EC 3.2.1.1) catalyzes the hydrolysis of starch to sugars acting on the α-1,4-glycosidic bonds in starch, glycogen and various oligosaccharides, releasing α-anomeric products. This enzyme has been studied extensively from various aspects: structure and function, secretion, and industrial application. The three-dimensional X-ray structures have been reported for α-amylases from Aspergillus oryzae, (AOA),31 A. niger,32 Bacillus licheniformis.33 Despite differences in their amino acid sequences, they have similar three-dimensional structures with three domains: domain A consisting of a central and typical parallel-stranded alpha-beta barrel structure, (β/α)8 barrel flanking the active site, domain B overlaying the active site from one side and domain C consisting of a β-structure with a Greek-Key motif or forming a “beta sandwich”. α-Amylases have conserved two aspartic acids and one glutamic acid, which are considered the catalytic residues. Site-directed mutagenesis experiments on the presumed catalytic residues showed that the substitution of any of these residues caused almost complete loss of activity and suggested that all the three residues were important for activity.34
The catalysis mechanism of α-amylase requires specific site groups to occupy a certain protonation state. That is, a certain number of amino acids in the active site must either be positively charged, neutral or negatively charged for the enzyme to function. Once the three-dimensional structure of an enzyme is known, then the dependence between measurable properties of an enzyme can be exploited, namely the pKa values of its ionizable residues directly involved in the catalytic region. The pKa values of these ionizable residues of the protein represent an attractive alternative to the rate constant. The pKa values are a measure of the chemical reactivity of ionizable residues and determine the pH-dependence of the rate for many enzymes. The pKa value of a given residue depends on the local protein geometry surrounding it.35 Thus, the measurement of pKa values can be predicted based on the protein structure using the program PROPKA.36 The pKa values predicted for the conserved three active site residues: two aspartic acids and one glutamic acid for AOA α-amylase (TAKA AMYLASE, Protein Data Bank code 6TAA): Asp206, Glu230, Asp297 (Figure 1A) and for bacterial α-amylase (Alteromonas haloplanctis bacterium α-amylase, Protein Data Bank code 1BLI): Asp231, Glu261, Asp328 (Figure 1B).
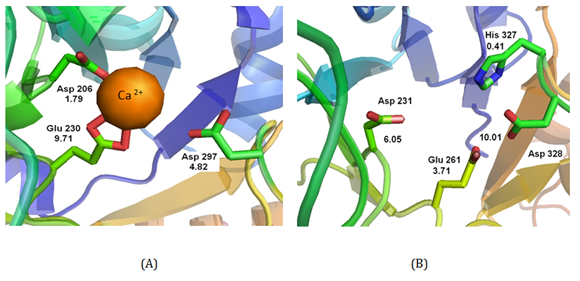
Figure 1 (A) Schematic drawing of the active site of AOA amylase (TAKA AMYLASE) (Protein Data Bank code 6TAA) showing the catalytic residues Asp206, Glu230, Asp297, Ca2+ cation and their corresponding pKa values. (B) Schematic drawing of the active site of IBA amylase (Alteromonas haloplanctis bacterium) (Protein Data Bank code 1BLI) showing the catalytic residues Asp231, Glu261, Asp328 and their corresponding pKa values.
Table 1 shows a summary of theoretical and experimental pKa values for both α-amylases. In the case of the structure of AOA α-amylase active site, the presence of a Ca2+ cation makes two contacts with OE1 and OE2 of Glu230; and a single contact with OD1 of Asp206 with distances of 2.71, 2.85; and 2.56 Å, respectively. Glu230 and Asp206 have pKa values of 9.71 and 1.79. The pKa values shifted up in both directions promoting the neutral state in the active site. The pKa value for Asp297 has a value of 4.82 (Figure 1A). For the bacterial α-amylase IBLI structure, there is no Ca2+ at the active site. The theoretical pKa values for Asp231, Glu261 and Asp328 are 6.05, 3.71 and 10.01, respectively. The higher pKa value for Asp328 may be due to the presence of His327 which is protonated with a pKa value of 0.41 and a relatively short distance of 3.52Å between NE2 of His327 and OD1 of Asp328.
|
Amylases |
Amino acid residue |
Theoretical pKa values |
Average pKa valuesa |
Average pKa valuesb |
|
|
Montecillo 2 |
Montecillo 3 |
||||
|
Asp206 |
1.79 |
||||
|
AOA |
Glu230 |
9.71 |
5.44 |
5.58 |
5.63 |
|
Asp297 |
4.82 |
||||
|
Asp231 |
6.05 |
||||
|
IBA |
Glu261 |
3.71 |
6.59 |
6.1 |
6.3 |
|
Asp328 |
10.01 |
||||
Table 1 Summary of theoretical and experimental pKa values for AOA and IBA amylases.
atheoretical and bexperimental pKa values, respectively
The pH-dependence of the protonation of amino acid residues are governed by their pKa values, that is, proteins can modulate the pKa values of amino acids in the active site by placing them in an immediately environment that favors either the charged or the neutral form of the amino acid. The AOA α-amylase (TAKA AMYLASE) contains a Ca2+ cation. The presence of this cation modifies ionization of the local environment at the catalytic site. The average pKa values involving the triad active sites residues for AOA and Alteromonas haloplanctis bacterium α-amylases are 5.44 and 6.59. From the pH-activity profile of AOA and bacterial α-amylases (IBA) using sorghum (Montecillo 2 and 3) hybrids starches as substrates, the pKa values were determined using the Dixon-Webb method (Figure 2).30 The pKa values determined for AOA and IBA α-amylases using sorghum (Montecillo 2 and 3) hybrids starches as substrates are 4.75, 6.4; 4.95, 6.3 with average pKa values of 5.58 and 5.63; and 4.8, 7.4; 5.0, 7.6 with average pKa values of 6.1 and 6.3, respectively. These average pKa values are in good agreement with the predicted values of 5.44 and 6.59 based on the protein structure using the program PROPKA (Table 1).36
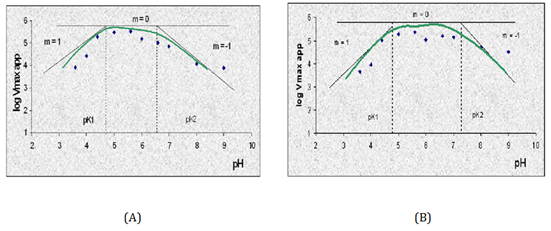
Figure 2 pKa profiles, (A and B) for the system AOA and IBA α-amylases using sorghum (Montecillo 2) as substrate. (C and D) for the system AOA and IBA α-amylases using sorghum (Montecillo 3) as substrate.
Nielsen et al.37 analyze pH-activity profiles in order to study the role of electrostatics in the active site of Bacillus licheniformis α-amylase by constructing site-directed mutants that were predicted to change the pKa values of the catalytic residues and thus change the pH-activity profile of the enzyme. In this paper, we determined the pKa profiles experimentally and carried out a comparison with predicted pKa values determined by using the program PROPKA. Our study indicated that the average pKa values experimentally and predicted are in good agreement.
This result agrees with the observation that ionizable groups with unusually low or high pKa values tend to occur at protein active sites.38–40 Starchy substances constitute the major part of the human diet for most of the people in the world, as well as many other animals. They are synthesized naturally in a variety of plants. Some plants examples with high starch content are corn, potato, rice, sorghum, wheat, and cassava. This paper describes the extraction of two varieties of white sorghum (Montecillo 2 and 3) and their hydrolysis by two α-amylases, namely a fungal α-amylase, AOA and the other an industrial bacterial type α-amylase (IBA). Both systems are characterized base on their pH and pKa profiles. Their average pKa values are in good agreement with the predicted values based on the three-dimensional protein structure information.
None.
The authors declare no conflicts of interest.

©2018 Soriano-García, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.