Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 1
Correspondence: Andréia HM, Department of Pharmaceutics, School of Pharmaceutical Sciences, University of São Paulo State (UNESP), Rod, Araraquara-Jaú, km 01, s/n, Campus Ville, Zipcode 14800-903, Araraquara-SP, Brazil, Tel +55 16 33016960, Fax +55 16 33016960
Received: January 02, 2018 | Published: January 24, 2018
Citation: Moreno AH, Lucchesi MBB, Dib SA, Salgado HRN (2018) Development of a New HPLC Method for the Determination of Lispro and Glargine Insulin Analogues in Pharmaceutical Preparations. J Anal Pharm Res 7(1): 00195. DOI: 10.15406/japlr.2018.07.00195
A rapid, accurate and sensitive HPLC method has been developed and validated for the quantitative determination of lispro and glargine insulin analogues in pharmaceutical preparations. Pharmacopeial method for the determination of insulin lispro employed mobile phase constituted by phosphate buffer solution 0.1mol/L (pH 2.3) and acetonitrile (74 + 26, v/v) and flow rate of 0.8mL/min. Retention time was estimated to be 25 minutes and the temperature of the column maintained to 40ºC. No pharmacopeial method was found to the determination of insulin glargine. For this reason, the aim of this study was to develop and validate a new chromatographic method for the determination of lispro and glargine insulin analogues in pharmaceutical preparations. Solutions were prepared using the mobile phase (methanol-water, 70:30) as solvent and filtered through a 0.2 µm membrane. Aliquots of 20µL were injected into the HPLC. The method validation parameters yielded good results and included range, linearity, precision, accuracy, specificity, and recovery. The calibration curves for lispro and glargine insulin analogues were linear from 0.1 to 3.5UI/mL, with correlation coefficients of 0.9990 and 0.9995, respectively. The interday and intraday precisions (relative standard deviation) were less than 1%. The accuracy was studied and the recovery test indicated mean absolute of 100.99% and 98.76% for lispro and glargine insulin analogues, respectively. The results obtained by HPLC method were calculated by analysis of variance (ANOVA). We concluded that the HPLC method proposed is satisfactory for the quantification of lispro and glargine insulin analogues in pharmaceutical preparations.
Keywords:insulin analogues, hplc method validation, quality control
HPLC, high-performance liquid chromatography; ANOVA, analysis of variance; UV, ultraviolet; LOD, limit of detection; LOQ, limit of quantification; CV, coefficient of variation; UI, international unit
Insulin is a polypeptide hormone synthesized by the beta cells of the lest of Langerhans in pancreas. It is the major anabolic hormone participating in the regulation of homeostasis.1–5 The insulin analogues are an altered type of insulin wich perform action as human insulin in terms of glycemic control.6–8 They are obtained by genetic engineering through alterations in the natural amino acid sequence of human insulin, changing its absorption, distribution, metabolism, and excretion.9–12 Lispro and glargine are types of insulin analogues (rapid and long-acting, respectively) commercialized for subcutaneous administration. In brazil lispro insulin is commercialized under the brand name Humalogâ, by Eli Lilly do Brasil, and glargine under the brand name Lantusâ by Sanofi-Aventis Ltda.13,14
Several analytical procedures are available in the literature for the analysis of insulin and its analogues. These methods are immunoassay,15–18 high performance liquid chromatography,19–25 and mass spectrometry.26–30 According to the pharmacopeial method for the determination of insulin lispro, the mobile phase is constituted by phosphate buffer solution 0.1mol/L (pH 2.3) and acetonitrile. Retention time (Rt) is estimated to be 25 minutes and temperature of the column maintained to 40ºC (19-23). No pharmacopeial method was found to the determination of glargine insulin.
The development of methods in HPLC for the determination of drugs has received considerable attention in recent years because of their importance in quality control in pharmaceutical analysis. Pharmacopeial assays still rely quite heavily on direct UV spectroscopy but in quality control the detection by UV spectrophotometry is usually combined with a preliminary separation by HPLC.31
Ideally, the buffer should transmit light at or below 220 nm so as to allow low-UV detection. All of the buffers, except citrate meet this criterion. However, buffer absorbance at low wavelengths can be strongly increased by the presence of impurities. Some buffers degrade on standing and may increase their UV absorbance during storage on long-term use, and are able to interact with the sample or the stationary phase by means of ion pairing. It should be noted that a change in buffer could result in a change in selectivity.32,33
In this work, the parameters developed for the determination of insulin analogues did not employed buffer, and the mobile phase (methanol/water, 70:30) used is simple and advantageous; the most quality control applications can be carried out with methanol/water as a mobile phase. For this reason, the purpose of the present work was to describe the development and validation of the simple and accurate HPLC method for the analysis of lispro and glargine insulin analogues in pharmaceutical preparations.
Insulin lispro (lot9074) and glargine insulin (lot73A) reference solutions with assigned purity of 100UI/mL as well the pharmaceutical preparations (injections) were generous donated by Eli Lilly (São Paulo, Brazil) and Sanofi-Aventis Ltda (São Paulo, Brazil). Insulin lispro injection was claimed to contain 100UI/mL of drug and glycerol, metacresol, sodium phosphate, zinc oxide and water as excipient. Insulin glargine injection was claimed to contain 100UI/mL of drug and glycerol, cresol, hydrochloric acid, sodium hydroxide, zinc chloride and water as excipient The reference solutions, as well as the pharmaceutical preparations (injections), were always kept protected from light and stored at refrigerator (8ºC).
Reagents and solvents
All other chemicals used were of pharmaceutical or special analytical grade. Distilled water purified using a Milli-Q system (Millipore, Milford, MA). All solutions were filtered through a membrane of 0.2 mm pore size (Hexis, Brazil).
Apparatus and chromatographic conditions
Quantitative HPLC was performed on a Waters Binary HPLC Model 1525 chromatograph equipped with a variable-wavelength detector (set at 214 nm) and injection valve with a 20mL loop. The analytical column was a C18 Wat 054275 (150 mm x 4.6 mm id, 5mm particle size) column. The mobile phase used was methanol/water (70:30, v/v). All analyses were done under isocratic conditions at a flow-rate of 1.0mL/min and at room temperature. The sensitivity was 0.5 AUFS and the chart speed 0.5cm/min(Table 1). The HPLC system was operated at ambient temperature (20 ± 1ºC). The mobile phase was degassed for 15 minutes and vacuum filtered through 0.2mm x 47mm filtration membrane (Hexis, Brazil). The analysis required 5 minutes.
|
Condition |
Method selection |
|
Mobile phase |
Methanol: Water (70:30) |
|
Column |
Waters C18 (WAT 054275) |
|
Wavelength |
214nm |
|
Flow rate |
1.0mL/min |
|
Detection |
0.5AUFS |
|
Injection volume |
20.0µL |
|
Temperature |
22º ± 1ºC |
Table 1 HPLC conditions for determination of insulin lispro and insulin glargine in pharmaceutical preparations.
Procedure
Preparation of lispro and glargine insulin analogues (reference solutions): Aliquots of 5.0mL of the lispro and glargine insulin analogues reference solutions (100UI/mL) were transferred to 50mL volumetric flasks and the volume was completed with the mobile phase (10.0UI/mL). So, aliquots of 0.1, 0.5, 1.0, 2.0, 3.0 and 3.5mL of the stock solutions (10.0UI/mL) were transferred to 10mL volumetric flasks, diluted to mark with the mobile phase and filtered, yielding concentrations of 0.1, 0.5, 1.0, 2.0, 3.0 and 3.5UI/mL for the calibration curves. The final concentration investigated was 2.0UI/mL, and 20µL were injected.
Assay of lispro and glargine insulin analogues (pharmaceutical preparations): Aliquots of 5.0mL of the lispro and glargine insulin analogues (100UI/mL) were transferred to 50mL volumetric flasks and the volume was completed with the mobile phase (10.0UI/mL). So, aliquots of 0.1, 0.5, 1.0, 2.0, 3.0 and 3.5mL of the stock solutions (10.0UI/mL) were transferred to 10mL volumetric flasks, diluted to mark with the mobile phase and filtered, yielding concentrations of 0.1, 0.5, 1.0, 2.0, 3.0 and 3.5UI/mL for the calibration curves. The final concentration investigated was 2.0UI/mL, and 20µL were injected. Peak areas of injected sample solutions were obtained and compared with peak areas from the standards. All determinations were conducted in triplicate.
Calculations
Having established the quantitative relationships among the parameters studied and knowing the predictive performance of their association model, a linear regression by the least squares method was applied.
Method validation
The method was validated by determination of the following operational characteristics: linearity, range, precision, specificity, and accuracy.34-36 The accuracy and precision of the assay, as well as the linearity of the calibration curve, were determined intraday and interday on 3 different days. The precision was expressed as the relative standard deviation (RSD, %) of each curve. The statistical data were calculated by analysis of variance (ANOVA).
Linearity: In order to assess the validity of the assay, amounts of 5.0mL of lispro and glargine reference solutions were diluted in 50mL volumetric flasks and final volumes were completed with the mobile phase (10.0UI/mL). Appropriate aliquots of this solution were diluted with mobile phase, yielding concentrations of 0.1, 0.5, 1.0, 2.0, 3.0 and 3.5UI/mL. Triplicate injections of each concentration were performed.
Limit of detection (LOD) and quantification (LOQ) values: The LOD and LOQ values were directly calculated by using the calibration line. The factors 3.3 and 10.0 for LOD and LOQ, respectively, were multiplied by the ratio from the residual standard deviation and the slope (corresponding to the standard error of the slope).
Precision: Repeatability was calculated by assaying 6 samples of the 100% standard concentration (2.0UI/mL). Intermediate precision was assessed by comparing the results obtained from 6 samples preparing on 3 different days.
Accuracy: Accuracy was evaluated by adding sample solutions (2.0UI/mL) to known concentrations of reference substance (0.1, 0.2 and 0.4UI/mL). Portions of 2.0 mL of the sample stock solutions (10.0UI/mL) were transferred to 10mL volumetric flasks to which 0.1, 0.2 and 0.4mL of the reference solutions (10.0UI/mL), equivalent to 0.1, 0.2 and 0.4UI/mL of the reference solutions, were added. After this procedure, dilutions were made with the mobile phase to give final concentrations of 105, 110 and 120% for R1, R2 and R3, respectively, of the sample concentrations used in the assay.
Robustness: Robustness was established by changing the chromatographic system parameters, such as column, flow rate, and mobile phase proportion.
The goal of this study was to develop an HPLC assay for the analysis of lispro and glargine insulin analogues in pharmaceutical preparations. For drug analysis in quality control, the simplest and fastest procedures are advantageous. In this study, the chromatographic conditions were influenced by the physicochemical properties of insulin analogues, such as solubility, polarity, and UV absorption. The described mobile phase was developed to provide a rapid quality control determination of lispro and glargine insulin analogues in pharmaceutical preparations. The wavelength of 214 nm was selected in order to permit the correct determination of drugs by UV detection. A sharp and symmetrical peak was obtained with good baseline resolution and minimal tailing, thus facilitating accurate measurement of the peak area ratio. The chromatogram of the sample peaks matched with the corresponding chromatogram of the standard drug peaks, which showed that the peaks of lispro and glargine insulin analogues were pure and also that formulation excipient were not interfering with the drug peaks (Figure 1).
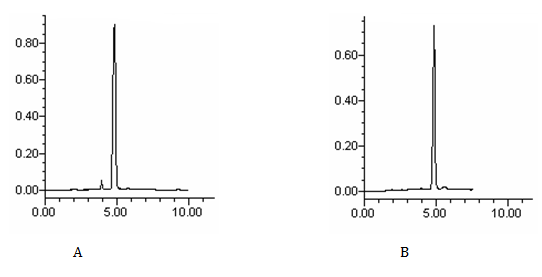
Figure 1 Chromatograms of insulin lispro (A) and insulin glargine (B) reference solutions by the proposed method using methanol/water (70:30) as mobile phase
The optimum mobile phase was obtained with methanol/water (70:30, v/v). This mobile phase allowed the elution of lispro and glargine insulins with adequate retention time. The repeatability of Rt during the precision studies was found to be excellent for all solutions. The Rt values of lispro and glargine insulins in reference solutions and pharmaceutical preparations were 4.8 minutes and 5.0 minutes, respectively (Figure 1). No interference from the sample excipient was observed at 214nm. Validation of the method was performed according to the AOAC International,34 the International Conference on Harmonization35 and Brazilian recommendations.36 HPLC conditions for determination of lispro and glargine insulins in pharmaceutical preparations are shown in Table 1. Linearity was studied by plotting concentration versus peak area; the calibration curve showed good linearity in a concentration range from 0.1 to 3.5UI/mL of drugs. The regression equations were calculated by the least-squares method. The representative linear equations were y = 324169x – 2024 and y = 301600x + 1491 for lispro and glargine insulins, respectively, where x is concentration of drug in UI/mL. The calculated LOD and LOQ values were 0.02UI/mL and 0.06UI/mL for insulin lispro, respectively, and 0.01UI/mL and 0.05UI/mL for glargine insulin, respectively. The correlation coefficients were bigger than 0.999 (Figure 2), and the CV was < 1% for lispro and glargine insulins.
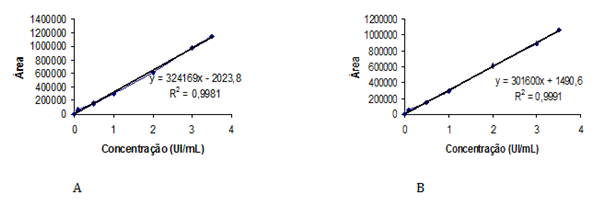
Figure 2 Calibration curves for insulin lispro (A) and insulin glargine (B), obtained by the HPLC proposed method.
The method was validated by evaluation of intraday and interday precision. In the range of 0.1-3.5UI/mL, the CV on the basis of the peak area ratios for 3 replicates injections were found to be between 0.08 to 1.98% for insulin lispro, and 0.07 to 1.03% for insulin glargine. The interday precision (3 days, n = 6) of the assay expressed as CV ranged from 0.55 to 0.59% for insulin lispro, and 0.56 to 0.79% for insulin glargine, respectively (Table 2 & 3). The mean absolute recovery determined by adding known amounts of reference solutions (0.1, 0.2 and 0.4UI/mL) to the samples was found to be 100.99% and 98.76% (lispro and glargine, respectively). The experimental values obtained for the determination of recovery are shown in Table 4 & 5. The CV of the symmetry and the peak area response were 2%. Recovery tests confirmed the accuracy of the proposed method.
|
|
Day 1 |
Day 2 |
Day 3 |
Mean inter-day |
|
I |
99.82 |
100.37 |
98.73 |
|
|
II |
98.94 |
100.40 |
99.96 |
|
|
III |
99.95 |
99.42 |
100.18 |
|
|
IV |
100.53 |
99.64 |
99.35 |
|
|
V |
100.31 |
98.88 |
98.82 |
|
|
VI |
99.88 |
99.74 |
99.24 |
|
|
Mean |
99.90 |
99.74 |
99.38 |
99.67 |
|
s |
0.5480 |
0.5828 |
0.5872 |
0.5727 |
|
CV |
0.5485 |
0.5843 |
0.5909 |
0.5746 |
|
n |
6 |
6 |
6 |
6 |
Table 2 Determination of insulin lispro in pharmaceutical preparations by the chromatographic proposed method in three different days.
s, standard deviation; CV, coefficient of variation; n, number of determinations
|
|
Day 1 |
Day 2 |
Day 3 |
Mean inter-day |
|
I |
99.32 |
98.86 |
99.82 |
|
|
II |
99.90 |
99.33 |
100.41 |
|
|
III |
99.61 |
99.11 |
99.21 |
|
|
IV |
100.42 |
99.98 |
98.38 |
|
|
V |
100.88 |
100.43 |
99.52 |
|
|
VI |
99.96 |
100.37 |
98.46 |
|
|
mean |
100.02 |
99.68 |
99.30 |
99.66 |
|
s |
0.5626 |
0.6717 |
0.7892 |
0.6745 |
|
CV |
0.5625 |
0.6739 |
0.7948 |
0.6771 |
|
n |
6 |
6 |
6 |
6 |
Table 3 Determination of insulin glargine in pharmaceutical preparations by the chromatographic proposed method in three different days.
s, standard deviation; CV, coefficient of variation; n, number of determinations
|
|
Added (UI/mL) |
Found (UI/mL) |
Recovery (%) |
CV (%) |
|
R1 |
0.1 |
0.1005 |
100.50 |
1.99 |
|
0.1025 |
||||
|
0.0985 |
||||
|
R2 |
0.2 |
0.2095 |
101.92 |
2.70 |
|
0.2035 |
||||
|
0.1985 |
||||
|
R3 |
0.4 |
0.4025 |
100.54 |
1.87 |
|
0.3945 |
||||
|
0.4095 |
Table 4 Experimental values obtained in the recovery test for insulin lispro in pharmaceutical preparations, by HPLC proposed method.
CV, coefficient of variation
|
|
Added (UI/mL) |
Found (UI/mL) |
Recovery (%) |
CV (%) |
|
R1 |
0.1 |
0.0987 |
99.37 |
2.09 |
|
0.1017 |
||||
|
0.0977 |
||||
|
R2 |
0.2 |
0.2037 |
98.85 |
3.32 |
|
0.1987 |
||||
|
0.1907 |
||||
|
R3 |
0.4 |
0.3877 |
98.07 |
1.03 |
|
0.3854 |
||||
|
0.3937 |
Table 5 Experimental values obtained in the recovery test for insulin glargine in pharmaceutical preparations, by HPLC proposed method.
CV, coefficiet of variation
Lispro and glargine insulin analogues in pharmaceutical preparations (injection) were analyzed, and the results obtained can be in Table 2 & 3. No interference from excipients could be observed at the detection wavelength (214nm), as shown in Figure 1. The method exhibited good robustness because the changes made in chromatographic conditions did not influence the analytical results. Lispro and glargine insulin analogues were shown to be stable during the procedure.
The present study developed a rapid and precise method for the determination of lispro and glargine insulin analogues in pharmaceutical preparations (injection) by HPLC. The method demonstrated acceptable linearity, sensitivity, precision, and accuracy. The method use simple reagents with minimum sample preparation procedures and short analysis time, encouraging its application in routine analysis. The results indicate that the proposed method might be recommended for the quality control of lispro and glargine insulin analogues in pharmaceutical preparations.
The authors are grateful to Eli Lilly (São Paulo, Brazil) and Sanofi-Aventis Ltda. (São Paulo, Brazil) for providing lispro and glargine reference solutions and pharmaceutical preparations. This work was supported by FAPESP (São Paulo, Brazil), Fundunesp (São Paulo, Brazil), CNPq (Brasília, Brazil) program, PACD-FCF-UNESP (Araraquara, Brazil) and CAPES-PROEX (São Paulo, Brazil).
The authors declare that there are no conflicts of interest.

©2018 Moreno, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.