Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 2
Correspondence: , Tel -254994
Received: October 20, 2015 | Published: April 11, 2016
Citation: Upmanyu N, Shah K, Porwal PK, Talele GS (2016) Degradation Kinetics of Olopatadine HCL Using a Validated UV-Area under Curve Method. J Anal Pharm Res 2(2): 00016. DOI: 10.15406/japlr.2016.02.00016
The objectives of this investigation were to establish a validated UV-area under curve (AUC) method for assay of Olopatadine HCL and to study the degradation behaviour of the drug under different ICH-recommended stress conditions. The spectral measurements were performed using AUC; 275 ↔ 320 nm with wavelength maxima at 298 nm in 0.9% w/v sodium chloride as solvent. The method was validated for linearity, precision, accuracy, robustness, specificity, and sensitivity, using sample of the bulk drug. Linear regression analysis data for the calibration plots showed a good linear relationship between response and concentration in the range of 5-100µg/mL. The drug was subjected to mild and moderate forced degradation and the degradation constant was calculated using linear as well as non-linear fit model for all the conditions. The drug was found to be less stable in acidic and photolytic degradation conditions. Photolytic degradation constants of Olopatadine in dry and wet state were 0.002488 and 0.003583 % min-1, respectively. The effect of deliberate changes in solvent strength and upper and lower wavelength were observed on AUC using 33 fractional factorial designs. The overall % RSD for robustness studies was 1.15. The utility of the method was verified by analyzing the drug in bulk form and a marketed formulation, the assay was found to be 99.04-101.5% and 99.15-99.27%, respectively. These results indicate the method can be successfully used for routine analysis of Olopatadine HCL in the bulk drug and in pharmaceutical dosage forms.
Keywords: olopatadine HCL, UV-area under curve, degradation rate constant, fractional factorial design, ICH validation
Olopatadine HCL {OP; (Z)-11-[3-(Dimethylamino) propylidene]-6, 11-dihydrodibenz [b, e] oxepin-2-acetic acid Hydrochloride} is a relatively selective H1-receptor antagonist and an inhibitor of histamine release from the mast cell.1 It is specifically formulated for the topical administration to the eyes. Ophthalmic solution of OP has been recently added in the revision bulletin of the United State Pharmacopoeia2 and Japanese Pharmacopoeia.3
Estimation of OP using UV-method, based on dye formation of the drug with an acidic dye, has been reported in literature.4 The procedure seems to be cumbersome and expensive without advantage in sensitivity. In another study, validation of UV-visible Spectrophotometric assay methodhas been performed for the estimation of OP using 206nm as its wavelength maxima.5 The drug does not show spectral homogeneity at the corresponding wavelength. Moreover, validation of the method was reported by using magnesium as an assumed placebo which can never be a part of ophthalmic preparation. Another reported assay method,6 based on UV-derivative spectroscopic technique, claimed, its estimation in marketed formulation. Surprisingly, the placebo (i.e. Benzalkonium Chloride) strongly absorbs in the same region as that the drug; a fact which has been overlooked in the study.
A stability indicating RP-HPLC-UV assay method for estimation of OP in tablet dosage form has been reported in literature.7 OP is zwitterionic in nature; therefore, it would be ionized at the mentioned pH (i.e. 4.5) and none of the functional group will maintain unionized state.8 The retention in such case is actually governed by high lipophilicity of OP (log p>4.0), which results in non-robust and weak RP-HPLC method.
Additionally, few hyphenated techniques as HPLC-MS/MS for determining the drug from human plasma,9-11 and for analyzing its stability sample12 are reported. Using these methods for routine analysis becomes very expensive and moreover, hyphenated equipments are not available usually. The drug is claimed to be “photosensitive” in a product monograph13 of ophthalmic preparation (PATANOL®; 0.1% w/v OP) and confirmed by a separate stability indicating HPLC-MS study.12
Spectrophotometric methods are widely used in analytical chemistry for their easy interpretation of the spectral data and simplicity in operating conditions. Nevertheless, the traditional spectrophotometric methods use of wavelength maxima those are not enough to furnish necessary information to resolve sample with components overlapping spectra.14 The fundamental quantity in spectroscopy is not the amplitude but the Area under Curve (AUC).15
In UV-Vi Eq (1)sible spectroscopy a Gaussian curve, is usually assumed using data normalized to a molar solution in a 1cm path-length as given in equation 1
(1)
Where, Aλ and Am are the molar absorbance’s at λ and λo, respectively. As Γ is the half-width at 0.5Am and Δ is the half-width when A has decreased to 0.37 Am (i.e. Am/e). Therefore, Δ will be equal to 1.2*Γ.
Thus, integrated area of the Gaussian curve can be obtained by using equation 2.
(2)
No study so far has been reported on the degradation kinetics models of OP in various stress conditions suggested by ICH Q1A (R2).16 Therefore, the present study was conducted considering general interest. The study described the development and validation of a simple, specific, sensitive, accurate and precise stability indicating UV-AUC method for the determination of OP in bulk drug and finished formulation as per ICH Q2 (R1).17 Forced degradation studies of OP were exercised in acidic, basic, oxidative and neutral hydrolytic environment. Thermal and photo stress studies were performed in wet conditions in addition to dry conditions as the drug is marketed in the form of solution. Degradation kinetics models of OP were generated on the basis of its degradation behaviour. The developed method was successfully for estimation of OP in bulk drug and finished formulation.
Chemicals and reagents
Gift sample of Olopatadine HCL was generously provided by Mylan Pharmaceutical Ltd. (Nasik, India). Quinine mono Hydrochloride dehydrate (claimed purity of 99.0%) used here as a standard for determination of sunny day, was purchased from Loba Chemie Pvt Ltd (Mumbai, India). Marketed preparation of OP (Winolap®, label claim 0.1% w/v or each ml was containing 1mg of OP) was procured from the retail pharmacy. Sodium chloride, 85% ortho-phosphoric acid (H3PO4) and Methanol AR grade of analytical-reagent grade were purchased from the same source. All buffers and solutions were prepared with purified and deionized water. Unless otherwise specified, all solutions were filtered through a 0.45 µm Ultipor®Nylon - 66 membranes filter (Pall Life Sciences, USA) prior to use.
Instruments and software
An UV-Visible spectrophotometric system (JASCO V-630, Kyoto, Japan) and a pair of Quartz cell Q-244 (Optiglass, Britain) of 1cm path length were used for the quantitation of sample. Spectroscopic data were recorded and processed using System-manager® software (JASCO, Kyoto, Japan). For statistical calculation of degradation rate constant in stability studies and experimental design in robustness study Graphpad PRISM®version 5.1 for Windows, (Graphpad software Inc., California, USA) software and Design-Expert 8.0.7.1 demo version software (Stat-Ease Inc., Minneapolis, MN, USA), were used, respectively.
Spectroscopic conditions and solvent optimization
Solubility behaviour18 of OP was analysed in water, methanol, 0.1N Sodium Hydroxide (NaOH), 0.1N Hydrochloric acid (HCL), 0.9% (w/v) Sodium Chloride (NaCl) solution and combinations of water: methanol. To avoid any ambiguous effect all the experiments were performed in dim light. Standard solutions containing low, medium and high amount of OP, covering the absorbance range suggested by Beer Lambert’s law,20 were prepared in the above mentioned solvents and optimal absorbance wavelength were selected after carrying a UV-scan over 400-200 nm. Optimal absorbance of OP at 299 nm and AUC of 275↔320 nm (peak valley ↔ peak valley; excluding baseline) were selected as spectroscopic units for calculation. Each solution was performed in duplicate, controlling the relative standard deviation (RSD) for peak AUC275↔320 below 2.0%, as required. Graphs, for various solvent systems were plotted between absorbance verse concentration and AUC verse concentration and standard error for y-intercept and slope were calculated. Solvent system having the least standard error for y-intercept and slope was chosen as diluent media for OP.
Preparation of the calibration curve standard solutions
A stock solution of OP (1000µg/mL) was prepared in 0.9% (w/v) NaCl solution (diluent) and was stored at 2-8ºC in dark until used. The solution was prepared by dissolving 100mg of OP in 60 mL of diluent and in a 100 mL volumetric flask. Vigorous hand shaking for about 2 min followed by sonication for 5min was performed to make the drug completely dissolve. Volume was made up to 100 mL with diluent. Aliquot of stock solution was diluted stepwise with the diluent to obtain 50µg/mL of OP. This solution was used for the optimization of the proposed method. A diluted standard solution containing 20µg/mL of OP was prepared by diluting suitable quantity of stock solution with solvent medium.
The calibration curve standard solutions containing 5, 10, 20, 30, 40, 50, 60, 80, 100µg/mL of OP were prepared by serially diluting stock solution with solvent medium. Calibration curve was drawn by plotting the peak AUC275↔320 of OP versus its corresponding concentration. The process was repeated twice (three consecutive days) using the same concentration range. Values for % RSD, slope and Y-intercept of the calibration curve were calculated.
Specificity
The specificity was performed in two parts namely specificity part-A and specificity part-B. In specificity part-A interference due to blank and placebo in absorption region of OP, was checked. The peak AUC275↔320 of OP in a spectrum obtained with diluted standard solution was compared with the spectrum obtained by analyzing solutions that contained excipients (0.1% w/v benzalkonium chloride and phosphate buffer) and a blank solution, under similar conditions.
In Specificity part-B, sample was subjected to various stress conditions,16 viz .different levels of acidic hydrolysis, alkaline hydrolysis, neutral hydrolysis, and oxidation conditions at a concentration of 1mg/mL. The finished pharmaceutical preparation of OP is marketed in the form of solution; therefore, the drug was subjected to thermal and photo-degradation in dry as well as in wet state. Quinine chemical actinometry was performed to justify the intensity of sunny day.19
The peak AUC275↔320 was observed in each spectrum and the results were fitted to linear as well as non-linear models. The degradation rate constant for every stress condition was determined using best fit model of one-phase exponential degradation plot using PRISM®statistic software.
Linearity and range
Three replicates of each of five dilutions, whose concentration was within the range 80-120% of the work-concentration (40, 45, 50, 55 and 60µg/mL) were prepared by diluting MET stock solution with mobile phase. The experimental results are represented graphically by obtaining a calibration graph between peak AUC275↔320 and concentration and carrying out the corresponding statistic study (ANOVA).
Precision
Precision of the study was tested at three different levels. The first consisted of checking instrumental system precision, where a sample corresponding to concentration of 50µg/mL was performed six times consecutively into the system, repeating the operation once again the next day. The second test consisted of checking the precision of the method, operating as described in the standard solution: six individual samples were prepared and the RSD was studied for the peak area obtained. Third, the intermediate precision was studied by preparing samples according to the precision of method and studying variability in the method when it is used by different analysts on different days.
Accuracy
Recovery of the method was studied at concentration levels of 80% (three samples), 100% (six samples) and 120% (three samples), whereby a known amount of the OP standard solution of 100% working concentration was added to a determined OP concentration levels of 80%, 100% and 120% in matrix solution containing 0.1% (w/v) benzalkonium chloride and phosphate buffer and the recovered OP concentration was calculated in relation to the added amount. The mean recovery for the method and the % RSD were calculated.
Limit of detection (LoD) and Limit of quantification (LoQ)
To determine the limits of detection and quantitation, the first point was to prepare a series of different concentrations by means of diluting a stock solution. Next, a calibration curve was established, where 100 % of the work concentration corresponded to 50µg/mL of OP. The concentration of all solutions ranged from 0.5 to 10.0µg/mL. Once, the results of the first linearity test were obtained, the response factor, i.e. the relationship between the AUC and the concentration was calculated for each of the points studied. From here on, the mean peak AUC, S.D. and RSD were calculated for each concentration. Subsequently, the concentrations in relation to the RSD obtained of the peak areas from each of the concentrations were represented graphically. The first point which conforms to the RSD corresponded to the detection limit, and the first point that actually matched corresponded to the quantitation limit.
Stability in analytical solution
This evaluation determines the period of time a solution can be held before analysis without compromising accuracy. The OP standard solutions were kept for 48 hours at bench top (≈25°C) in the dark as well as in the refrigerator (≈2-8°C), and their spectrum was taken. OP standard solution and sample solution (OP and placebo solution) containing test concentration were prepared in duplicate from fresh stock solution, and their spectrum was taken. Peak AUC was measured and the mean was calculated.
Robustness
Robustness of the method was checked with little deliberate variations in the method,25 like change in peak AUC start point (±2nm), change in peak AUC end point (±2nm) and change in strength of solvent medium (±2% relative).
A 27-run, 33 factorial designs, consisting of 3 factors at 3 levels, was set up to evaluate robustness of the spectroscopic conditions which are likely to be employed. Peak AUC start point (A), peak AUC end point (B) and strength of solvent medium (C) as per 33 factorial design are represented in Table 1. The effect of deliberate change on response factor was evaluated in duplicate. Therefore, the actual number of runs was 54. The targeted response parameters were statistically analyzed by applying one-way ANOVA at 0.05 levels in Design-Expert® statistic software. The individual parameters were evaluated using the F-test and mathematical models of the form indicated in Equation (3) were generated for each response:
|
Factor |
Name |
Unit |
Coded |
||
|
Lower (-1) |
High (1) |
Medium (0) |
|||
|
A |
Lower nm |
nm |
273 |
277 |
275 |
|
B |
Upper nm |
nm |
318 |
322 |
320 |
|
C |
Solvent Strength |
% |
0.88 |
0.92 |
0.9 |
Table 1 Constraints set on each variable with minimum and maximum coding
Type: Numeric
Sub-type: Discrete
(3)
Where, Y is the level of the measured response, β0 is the intercept, β1 to β9 are the regression coefficients, A, B and C stand for the main effects, AB, AC and BC are the two-way interactions between the main effects. The polynomial models containing only the significant terms (P<0.05) were generated for each response parameter using multiple linear regression analysis (MLRA) and analysis of variance (ANOVA).
The models generated were used to construct 3-dimensional graphs in which response parameter Y was represented as a function of variable factors A, B and C. The effect of independent variables on each response was also visualized from the contour plots. The effect of overall deliberate change was calculated in terms of % RSD to define robustness of the validated method.
A numerical optimization technique employing the desirability approach was used to locate the optimum spectral conditions for the UV-AUC method developed and hence to verify the mathematical models generated. Various feasibility and grid searches were executed to establish the robustness of spectral conditions for the method employed. Constraints set on each factor to locate the optimum spectral conditions are listed in Table 1. The optimized conditions were employed and evaluated for the responses. The experimental values of peak AUC was compared with those predicted by the mathematical models.
Application of the validated method
Laboratory mixture
The validated method was applied to a known laboratory solution of OP. A series of laboratory solutions containing 8.0, 10.0 and 12.0µg/mL of OP were prepared and concentration of the analyte was back calculated. The % error was calculated as per the equation 4.
(4)
Assay of OP in marketed eye drop formulation
Marketed eye drop containing OP (1mg/mL) were taken and diluted to get a concentration of 50µg/ml with 0.9% (w/v) NaCl solution. Each of the solutions was prepared in triplicate and spectrum was recorded and per cent assay was calculated using equation 5.
(5)
The UV- spectrum of OP shows two peaks viz. 208-212nm and 296-300nm. Earlier publications had calculated the spectral intensity in wavelength range of 208-212nm5,6 because aborptivity for peak apex in the range 208-212nm is more as compare to peak apex in the range 296-300nm. In the present work calculations were made for the spectral intensity of OP at 298nm, as the peak corresponds to 298nm was showing a Gaussian pattern along with good spectral homogeneity and recommended by Japanese pharmacopoeia3 also. Aside that, Spectrum calculations were also performed for peak AUC275↔320 of peak valley from 275nm to 320nm correspond to λmax 298nm of OP.
Olopatadine is zwitterionic in nature (Figure 1) and represented by two pKa values i.e., due to carboxyl group (HA; 4.29) and tertiary amino group (BH+; 9.19), therefore, the solubility of OP was checked in 0.1N HCL, 0.1N NaOH, de-ionized water and MeOH to get optimum response.
The solubility behaviour of OP was also analyzed in 0.9% (w/v) Sodium Chloride (NaCl) solution and combination of water: methanol. The drug was slightly soluble in water, which was reflecting its lipophilic nature (log p; 4.67). Though the solubility of OP was more in MeOH as compare to other solvent systems, but 0.9% (w/v) NaCl was chosen as solvent to dissolve OP. The selected solvent media is the major diluent for marketed formulation of OP and, thus, representing more real time approach as compare to MeOH, aside that, analyte has shown optimum absorbance and least standard error for y-intercept in the selected dissolution media (data shown in Table 2).
|
Solvent Medium |
Y-intercept±SD |
Slope±SD |
Regression Coefficient |
Sy. x |
|
Water |
0.003970±0.007562 |
0.007796±0.0001469 |
0.995 |
0.01976 |
|
0.1 N NaOH |
0.02026±0.01808 |
0.01089±0.0003512 |
0.986 |
0.04724 |
|
0.1N HCl |
0.02652±0.004934 |
0.007669±0.00009584 |
0.998 |
0.01289 |
|
Methanol |
0.02550±0.02346 |
0.01251±0.0004558 |
0.983 |
0.0613 |
|
Water: Methanol (20:80) |
0.05015±0.02433 |
0.01107±0.0004727 |
0.976 |
0.06358 |
|
0.9 % NaCl in Water (w/v) |
0.003207±0.001899 |
0.01289±0.00003688 |
0.999 |
0.004961 |
Table 2 Comparison of line properties of OP in different solvent medium at P< 0.0001
The drug had shown good linear relationship between wide concentration range and AUC275↔320 in 0.9 % NaCl in water (w/v). The line equation for the linearity curve is y=0.09194 (± 0.001011) x - 0.03804 and correlation coefficient r2 was 0.999.
For the current method 50µg/mL was taken as test concentration because it can be serially prepared from the stock solution (1000→100→50µg/mL) and having optimum response (~5.47) at AUC275↔320.
Specificity
Spectra were recorded separately for blank, placebo and diluted standard containing 20µg/ml of OP and displayed non-overlapped, peak area (AUC275↔320)for OP. Overlaid spectra of blank, placebo and OP are shown in Figure 2. Benzalkonium chloride, a preservative commonly used in eye drop formulations, shows absorption in the UV-region. The wavelength maximum for benzalkonium chloride was 256nm and corresponding peak’s valley was observed at 267nm. Thus, the UV- AUC275↔320 method presented in this study is selective for OP.
The forced degradation studies of OP were performed under various stress conditions recommended by ICH guideline16 and decision tree available in literature.21 Though the UV-Vis spectroscopy is not a preferred technique to validate stability indicating assay methods and it cannot be applied as an option for chromatographic separation but the UV-AUC could be used to calculate some other application of an assay method viz. calculation of degradation rate constant, content determination in stability samples, re-testing of storage sample etc. as in the mentioned method spectral intensity is calculated via selection of multiple wavelengths and integration of area underlying those wavelengths.
The results of specificity part-B (forced degradation studies) of OP, using finally developed method, suggested the following degradation behaviour; results are tabulated in Table 3.
|
Stress Condition |
Degradation Constant (k; % min-1) |
Half Life (in min) |
Correlation Coefficient |
Absolute Sum of Squares |
Sy. x |
|
0.1N HCl |
0.005149 |
134.6 |
0.9377 |
122.6 |
3.914 |
|
1.0N HCl |
0.0361 |
19.2 |
0.8731 |
201.2 |
5.014 |
|
0.1N NaOH |
0.001121 |
618.6 |
0.9681 |
4.323 |
0.7351 |
|
1.0N NaOH |
0.01033 |
67.13 |
0.9657 |
9.876 |
1.111 |
|
3.0% H2O2 |
0.00708 |
97.91 |
0.9877 |
26.26 |
1.812 |
|
30% H2O2 |
0.006135 |
113 |
0.9867 |
66.09 |
2.874 |
|
Neutral (H2O) |
~ 2.689e-005 |
~ 25775 |
0.9834 |
0.2601 |
0.1803 |
|
Thermal (Dry) |
0.01400 (%hr-1) |
49.51(hr) |
0.9443 |
19.43 |
1.8 |
|
Thermal (Wet) |
0.04753(%hr-1) |
14.58 (hr) |
0.9938 |
7.297 |
1.103 |
|
Photolytic (Dry) |
0.002488 |
278.6 |
0.8571 |
94.76 |
3.245 |
|
Photolytic (Wet) |
0.003583 |
193.5 |
0.986 |
19.4 |
1.468 |
Table 3 Degradation kinetics of Olopatadine HCL in different stress conditions
Degradation in acidic conditions
OP was observed to be degraded to about 75% in acidic conditions, when treated with 0.1N HCL for 1 hr at room temperature (≈25ºC). The degradation rate constant and half life for OP in 0.1N HCL were 0.005149 % min-1 and 134.6 min, respectively. The drug was following linear and one-phase degradation pattern in the case of 0.1N HCL. The degradation rate constant and half life for OP in 1.0N HCL were 0.03610 % min-1 and 19.20 min, respectively. The degradation pattern was ambiguous in the case of 1.0N HCL as drug had shown fast degradation at the initial time points (from 20 to 30 minute) followed by plateau phase as shown in the Figure 3a.
Degradation in basic conditions
The drug was found to be relatively stable in basic degradation conditions as compare to acidic degradation conditions. OP was observed to be degraded to about 95% in basic conditions, when treated with 0.1N NaOH as well as with 1.0N NaOH for 1 h at room temperature (≈25ºC). The degradation rate constant and half life for OP in 0.1N NaOH were 0.001121% min-1 and 618.6 min, respectively. The drug was following linear degradation pattern in the case of 0.1N NaOH. The degradation rate constant and half life for OP in 1.0N NaOH were 0.01033 % min-1 and 67.13 min, respectively. The degradation pattern was one-phase exponential decay in the case of 1.0N NaOH as shown in the Figure 3b.
Degradation under oxidative conditions
OP was observed to be degraded to about 90 % in mild oxidative environment, when treated with 3.0% H2O2 for 30 minute at room temperature (≈25ºC). The degradation rate constant and half life for OP in 3.0 % H2O2 were 0.007080 % min-1 and 97.91 min, respectively. The degradation rate constant and half life for OP in 30.0% H2O2 were 0.006135% min-1 and 113.0 min, respectively. The degradation pattern was one-phase exponential decay in both the cases of oxidative stress as shown in the Figure 3c.
Degradation in photolytic conditions
The determination of sunny day for photolytic degradation studies was done using quinine chemical actinometry method studied by ICH22,23 and USFDA24 working groups. The referred method was slightly modified in terms to dissolve it. The stock solution of Quinine was prepared in solvent medium containing water: methanol in the ratio of 90:10, respectively, instead of water alone. The day of photo-degradation study was declared to be “sunny” as the difference in the absorbance of control (AC) and test (AT) was 1.012 (Limit: ΔA should be more than 0.9).
OP was observed to be degraded to about 90% in wet state when exposed to sunlight. The photo-degradation was relatively high in wet state as compare to dry state. The degradation rate constant and half life for OP in wet state were 0.003583% min-1 and 193.5 min, respectively. Whereas, the degradation rate constant and half life for OP in dry state were 0.002488% min-1 and 278.6 min, respectively. The degradation pattern was linear in dry state and a fast one-phase exponential degradation pattern was observed in the case of wet state as shown in the Figure 3d.
Thermal degradation
OP was observed to be degraded to about 95% in dry state when exposed to 105ºC for 12 hours. The thermal-degradation was relatively high in wet state as compare to dry state. The degradation rate constant and half life for OP in dry state were 0.01400% hr-1 and 49.51hr, respectively. Whereas, the degradation rate constant and half life for OP in wet state were 0.04753% hr-1 and 14.58 hr, respectively. The degradation pattern was linear in dry state and a fast one-phase exponential degradation pattern was observed in the case of wet state as shown in the Figure 3e.
Degradation under neutral conditions
OP was found to have a negligible degradation of about 2.0% under neutral conditions (in water for 12 h at 25°C) as the degradation constant and half life were about 2.68×10-5% min-1 and 2.57×104 min, respectively. The degradation pattern was flat linear as shown in the Figure 3f.

Figure 3 Degradation pattern of OP in (a) acidic, (b) basic, (c) oxidative, (d) photolytic, (e) thermal and (f) neutral environment.
Linearity
Linearity curves for OP were examined in pure solutions as well as in the spiked placebo solution and it were found to be linear; correlation coefficients ≥0.999 in both the cases. Statistical treatment of the linearity data of OP shows a linear response between lower levels to highest level with a line equation Y= 0.1044 (± 0.001946) X + 0.2020 (± 0.09826). In addition, the analysis of residuals shows values randomly scattered around zero, which fits well within the linear model. The origin of linearity curve was within the lower and the upper limit of 95% that gives high degree of confidence to the value obtained for intercept which was less than 5% of area response at 100% level.
LoD and LoQ
LoD and LoQ, as a measure of method sensitivity, were provided for OP calculated by means of % RSD method. The LoD and LoQ for OP were 2.0 and 5.0µg/mL, respectively. The % RSD for LoD and LoQ were 13.24 and 6.98, respectively. From the results, it can be concluded that the proposed method is sufficient enough to quantify small quantity of OP in samples.
Precision and repeatability
The results obtained for repeatability studies and for intermediate precision are presented in Table 4. Values of % RSD for system precision and method precision of OP were 0.33 and 0.95, respectively. Intermediate precision has a % RSD below 1.5 which complies with the acceptance criteria.
|
Olopatadine Concentration (µg/ml) |
Repeatability |
Intermediate Precision |
|
|
System Precision |
Method Precision (Analyst-I) |
Method Precision (Analyst-II) |
|
|
50 |
5.44 |
5.44 |
5.51 |
|
50 |
5.46 |
5.38 |
5.56 |
|
50 |
5.44 |
5.41 |
5.61 |
|
50 |
5.43 |
5.51 |
5.55 |
|
50 |
5.48 |
5.48 |
5.45 |
|
50 |
5.45 |
5.39 |
5.41 |
|
Mean |
5.45 |
5.435 |
5.475 |
|
S.D. |
0.017889 |
0.051672 |
0.073916 |
|
%RSD |
0.32823 |
0.950728 |
1.350072 |
Table 4 Repeatability and intermediated precision studies of OP
Accuracy
The results are expressed as percent recoveries of the particular components in the samples. The percent recoveries of OP at 80,100 and 120% of the test concentration were 97.94, 99.43 and 101.49%, respectively. The mean % age recovery was 99.62 with % RSD value of 1.78.
Stability in analytical solution
The analytical solution of OP was found stable in bench-top (≈25ºC) as well as in refrigerated (≈2-8ºC) conditions. The % change in assay value of OP was about 1.0 in both of the storage conditions as shown in the Figure 4. From the data, it was concluded that standard and sample solutions may be used up to 24 hr after preparation.
Robustness
A set of preliminary studies were undertaken to establish the range of each variable with the aim of effect of deliberate changes on the UV-AUC assay method of OP in bulk drug and pharmaceutical preparations.
As discussed earlier, saline solution (0.9% NaCl in water) was preferred as solvent media over other solvent system to dissolve OP. The drug was less soluble in the selected solvent system; therefore, every sample was subjected to sonication for about 5 minutes. Thus, the probable process variables were strength of dissolving media and sonication time. The sonication time was not involved in the robustness study as increase in sonication time was not altering the assay value (% RSD; < 0.50 %).
Therefore, concentration variable of ±0.02% NaCl (w/v) in water was employed for process variable i.e. strength of dissolving media 0.9% NaCl (w/v) in water. The starting and ending point, corresponding to Gaussian peak having wavelength maxima of 298nm, was taken as system variables (±2 nm) related to the optimized assay method.
A 33 factorial design was employed to evaluate the robustness of assay method. The results of analysis of variance and mathematical models generated by regressional analysis are represented in Table 5.
|
Source |
Sum of Squares |
df |
F- Value |
p-value |
|
Model |
1.01 |
9 |
29.02 |
< 0.0001 |
|
A- Lower nm |
0.039 |
1 |
10.23 |
0.0026 |
|
B- Upper nm |
0.62 |
1 |
159.44 |
< 0.0001 |
|
C- Conc. of buffer |
0.13 |
1 |
33.73 |
< 0.0001 |
|
AB |
2.67e-004 |
1 |
0.069 |
0.7939 |
|
AC |
1.04e-006 |
1 |
2.70e-004 |
0.987 |
|
BC |
1.50e-003 |
1 |
0.39 |
0.5357 |
|
A2 |
0.033 |
1 |
8.55 |
0.0054 |
|
B2 |
0.079 |
1 |
20.41 |
< 0.0001 |
|
C2 |
0.11 |
1 |
28.37 |
< 0.0001 |
|
Residual |
0.17 |
44 |
3.86e-003 |
|
|
Lack of Fit |
0.013 |
17 |
7.82e-004 |
|
|
Pure Error |
0.16 |
27 |
5.80e-003 |
|
Table 5 Anova for response surface quadratic model for robustness testing
The Fisher F-test indicated that all the mathematical models generated for the response parameters were found to be significant (P>F less than 0.05). The Model F-value of 29.02 implies the model was significant. There was only a 0.01% chance that a “Model F-Value” this large could occur due to noise. The predictor model generated for the peak AUC upon transformation was found to be significant as shown in Figure 5a.
The polynomial models constitute the coefficients for the intercept, first order main effects and interaction effects. The sign and magnitude of the main effects signify the relative influence of each factor on the response. Regression equations (equation 6) of the fitted model:
(6)
The Fisher F test with a very low probability value (P>F = 0.0001) demonstrated a very high significance of the predictor model generated for the peak AUC (Y). The goodness of fit test suggested quadric model for source design. The goodness of fit of the model was checked by the adjusted determination coefficient (adjusted R2). The determination coefficient (R2 ) is a measure of the amount of reduction of variability of Y obtained using the regression variables A, B, C.
The value of the determination coefficient (R2 = 0.8558) was in reasonable agreement with the adjusted determination coefficient (adjusted R2 = 0.8263), which confirmed the high significance of the model. A relatively low value of standard deviation (S.D. = 0.062) confirmed the improved precision and reliability of the conducted trials.
The polynomial model generated for the peak AUC indicated that the deliberate change in peak starting wavelength or lower nm (P > F = 0.0026) and peak ending wavelength or upper nm (P > F = 0.0001) had significant positive influence on Y, whereas, concentration of dissolving media (P > F = 0.0001) had significant negative influence on Y.
The combined influence of A and B on Y is shown by the 3-dimensional plot represented in Figure 5b. The pattern of the contours (Figure 5c) reflects that peak AUC within the experimental constraints can be obtained using allowed deliberate changes proposed for the assay method. The overall % RSD of the peak AUC value was 1.15, which is reflecting that the assay method of OP is sufficiently robust.
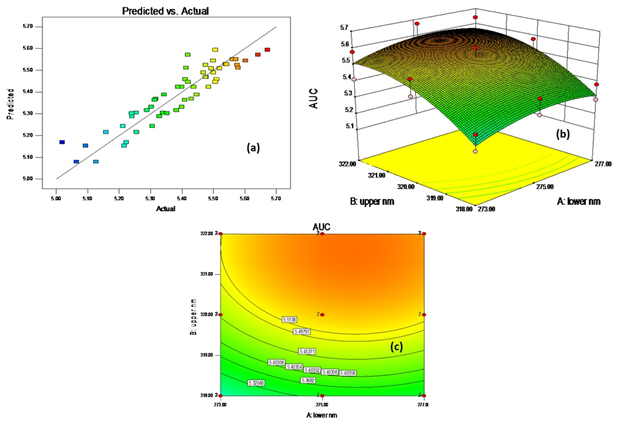
Figure 5 Graph representing (a) correlation for predicted verse actual value of peak AUC, (b) 3-D plot and (c) Contour plot showing the influence of deliberate changes on the peak AUC.
Application of the validated method
The developed method was applied to assay OP in bulk drug and marketed pharmaceutical preparation. The content was calculated as an average of three determinations and the experimental results are given in Table 6. The results were very close to each other as well as to the label value of commercial pharmaceutical preparation. Recoveries were very close to 100 %, which proved the suitability and accuracy of the proposed method.
|
Particular |
Labelled Amount |
Amount Found (n=3) |
Assay % |
Mean % Error |
|
Bulk drug |
40 µg/ml |
40.25 |
100.62 |
1.02 |
|
50µg/ml |
49.52 |
99.04 |
||
|
60µg/ml |
60.95 |
101.5 |
||
|
Marketed formulation |
1mg/ml |
0.991 mg/ml |
99.15 |
0.8 |
Table 6 Analysis of OP from bulk drug and pharmaceutical formulations by validated method
The proposed UV-AUC method for estimation of OP is analysed in bulk drug and marketed formulation as per ICH guidelines. The method is found to be selective and robust for the estimation of OP. The method is also stability indicating as evident from results obtained when method applied to forced degradation studies. The assay utilized a previously unreported set of conditions, including saline solution as solvent media and calculation of spectral intensity using peak-AUC. The proposed UV-AUC method validated using a 33 factorial design and optimized by employing the desirability approach provides a robust assay method. The method is found to be linear in the specified range, precise and sensitive. Accuracy of the method is also established for the formulation. Hence, the proposed method stands validated and may be used for routine and stability sample analysis.
None.
Authors declare that there is no conflict of interest.

©2016 Upmanyu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.