Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 4
Correspondence: Marjan Piponski, Quality Control Department of Replek Farm Ltd., st. Kozle 188, 1000 Skopje, Republic of Macedonia
Received: June 28, 2017 | Published: August 9, 2018
Citation: Piponski M, Stoimenova TB, Piponska M, et al. Concepts in development of fast, simple, stability indicating HPLC method for analysis of atorvastatin related compounds in tablets. J Anal Pharm Res. 2018;7(4):450-457. DOI: 10.15406/japlr.2018.07.00265
New, fast, simple and mild conditioned High Performance Liquid Chromatography (HPLC) method for determination of atorvastatin and its 7 main specified impurities, as well as unspecified impurities that might possibly appear, was developed. Chromatographic runs last between 25 and 40 minutes, with simple stepwise gradient elution. The main accent in our method development strategy was focused on mobile phase, composed of simple binary system composed of phosphate buffer and acetonitrile, at pH 4.1, without use of tetrahydrofuran, ion-pair reagents, trifluoroacetic acid and other modifiers with high Ultraviolet (UV) cut-off like absorptive acetate or formiate buffers or amines. With our concept of mobile phase, different columns from myriad were tested, with different efficiency, dimensions and properties, which resulted in different separation efficiency and run time. The best results, concerning essential critical peak resolution, run time length including column preparation and equilibration and column backpressure, were achieved with: YMC C18 Triart 150mm x 4.6mm, 3µm (YMC America, Inc.), afterwards with Nucleodur 100-3-C18ec 250mm x 4.6mm, 3µm (Macherey-Nagel GmbH & Co., Germany), Waters Symmetry C18 250mm x 4.6mm, 5µm (Waters, USA) and Superspher C18e 125mm x 4mm, 4µm (Merck, Darmstadt, Germany). All this columns achieve excellent results regarding obligated critical resolution between atorvastatin impurity B and atorvastatin (according to European Pharmacopoeia),1 or in some cases between atorvastatin impurity B and atorvastatin impurity C, to be minimum about 1.5, in both cases.
Keywords: atorvastatin, related compounds, HPLC, method, UV, optimization
Atorvastatin belongs to a group of drugs called 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors or “statins”, that reduce the level of “bad” cholesterol (Low Density Lipoproteins or LDL) and triglycerides in the blood, while increasing the level of the “good cholesterol” (High Density Lipoproteins or HDL). This drug is used to treat high cholesterol, to prevent the risk of stroke, heart attack or other cardiac complications in patients with type 2 diabetes, coronary heart disease and other risk factors. The IUPAC name of atorvastatin calcium (Figure 1) is (3R,5R)-7-[2-(4-Fluorophenyl-5-isopropyl-3-phenyl-4-(phenylcarbamoil)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid calcium salt (2:1).2
The search for adequate HPLC method for determination of atorvastatin and its related compounds is complicated by the scope of the analytical method application; is it going to be used for routine analysis by the quality control laboratories within the pharmaceutical industry, or the method will have additional research aim for atorvastatin related compounds. In the first case of routine analysis using of MS detectors is unnecessary and optional, whilst in second case it is an advantage.
Numerous HPLC analytical methods which describe simultaneous determination of atorvastatin in combination with other drug are published.3–12 If we focus on analytical methods for atorvastatin only, and we skip the methods using capillary electrophoresis, Ultraviolet-visible (UV-Vis) spectrophotometry and Liquid Chromatography (LC) with mass spectral detectors, LC methods with UV detector can also be found in published articles.13–19
The most important and generally accepted compendial method from European Pharmacopoeia (EP)1 for impurities testing of atorvastatin API, describes use of HPLC with UV signal monitoring at 244nm, octylsilyl C8 (L7) column with dimensions 250mm x 4.6mm and 5µm particles (recommended Zorbax C8 Rx as appropriate). This method describes combined elution starting with isocratic and followed with linear gradient mode of elution, with run time of 85 minutes, and mobile phases composed of acetonitrile, tetrahydrofuran and ammonium acetate buffer adjusted to pH 5.0 with glacial acetic acid, at flow rate 1.5ml/min.
The aim of our work was development of new, stability indicating HPLC method for determination of atorvastatin and its impurities that will possess the following advantages when compared to the already existing and used methods for the same purpose: shorter run times, simpler, cost-effective, using less toxic and more UV-transparent components in mobile phase composition. The main target for our new developed method are routine quality control laboratories in pharmaceutical industries as well as other types of pharmaceutical quality control laboratories worldwide, especially ones with modest or limited budgets for expenses, working with less performance chromatographic equipment and/or less experienced analysts.
In this research following LC systems were used: UPLC Dionex Ultimate 3000 controlled by Chromeleon version 6.8, UPLC Shimadzu Nexera controlled by Lab Solutions version 6.81, HPLC Varian ProStar controlled by Varian Star version 6.30 and Varian LC 920 controlled by Galaxy version 1.93. The organic chemicals acetonitrile and dimethylformamide were gradient grade, purchased from Merck, Darmstadt, Germany. Potassium dihydrogen phosphate and o-phosphoric acid were EP grade, also products of Merck, Darmstadt, Germany. The demineralized water used for analyses was in-house product of Stilman with conductivity less than 1µS/cm.
Standards of active substance atorvastatin and its four specified impurities: atorvastatin impuity A, atorvastatin impuity B, atorvastatin impuity C and atorvastatin impuity D (containing also atorvastatin impuity F, atorvastatin impuity G and atorvastatin impuity H) were supplied from EDQM. Atorvastatin impuity. F, atorvastatin impuity G and atorvastatin impuity H cannot be purchased form EDQM, but their presence in the chromatograms is suitably marked on the figures presented in this article, based on assumption considering European Pharmacopoeia referent chromatogram for atorvastatin calcium trihydrate and spectral characteristics of the obtained peaks.
The most valuable and successful separations were achieved with following chromatographic columns: YMC C18 Triart 150mm x 4.6mm, 3µm (YMC America, Inc.); Superspher C18e 125mm x 4mm, 4µm (Merck, Darmstadt, Germany); Waters Symmetry C18 250mm x 4.6mm, 5µm (Waters, USA); Nucleodur 100-3- C18ec 250mm x 4.6mm, 3µm (Macherey-Nagel GmbH & Co., Germany); LiChrospher C8, C8e, C18 and C18e and Purospher C8e STAR and C18e STAR, all with dimensions 250mm x 4mm, 5µm (Merck, Darmstadt, Germany); Hypersil C18 100mm x 4mm, 3µm (Thermo Scientific, USA); Discovery C8 and C18 and Supelco ABZ plus all with dimensions 150mm x 4.6mm, 5µm (Supelco Bellefonte USA); Spherisorb C8 and C18 ODS2 150mm x 4,6mm, 5µm (Waters, USA) and the pharmacopoeia cited Zorbax Rx C8 250mm x 4.6mm, 5µm (Agilent, USA).
The official method prescribed in European pharmacopoeia, current edition, for determination of impurities of atorvastatin calcium contains mobile phase composed of acetonitrile, tetrahydrofuran and acetate buffer, highly absorbing at the monitoring wavelength, 245nm. Additionally, tetrahydrofuran is highly toxic and expensive, requires longer column equilibration and is restrictive to types of components and salts that can be used in mobile phase composition. EP prescribed gradient elution is long lasting, with total duration time of 90 minutes, and typical chromatogram obtained using this method is presented in Figure 2.1
Most of the published methods are for simultaneous determination of atorvastatin in combination with many other drugs.3–12 There is also a large number of published HPLC methods for determination of atorvastatin alone13 or with its impurities,14–19 with UV detection. Two most applicative and interesting methods for testing atorvastatin impurities can be distinguished from the above mentioned, one from Petkovska et al.17 and the other from Vakkum et al.19 The publication from Petkovska et al.17 uses small high efficiency column with dimensions 50mm x 2.1mm and 1,8µm particles, with mobile phase containing acetonitrile, tetrahydrofuran and phosphate buffer with pH 3.5. Few details are little limiting factors, state of art column with 1.8µm which cannot be used with high dwell volume or high dispersion standard HPLC systems with lower performance characteristics, but only with UPLC systems with very low system dispersion volume, especially flow cell. These columns are more complicated for less skilled analysts and with higher probability of easier column clogging, thus limiting the maximum number of successful analyses. Moreover, this method uses tetrahydrofuran in the mobile phase, which we intended to exclude.
The second very interesting publication of Vakkum et al.19 uses simple inorganic part of mobile phase consisting of 0.1% v/v trifluoroacteic acid with gradient elution with acetonitrile. Separations seem very interesting and attractive. High resolutions are mentioned in tables with values up to 2, even though EP requires minimum of 1.5. But these high resolutions are achieved with peaks of related compounds present in low concentrations, at their detection limits, where peaks are always narrowest and easier to separate. Also, disadvantage is the low pH value of the 0.1% v/v trifluoroacetic acid, usually little less than 2, which is out of the recommended pH range between 2-9 of the cited column Zorbax Bonus RP. This will influence on a reduction of the approximate column life and use.
During our method development, the focus was directed towards investigations within the two attributes which describe the best the chromatographic process: selective and simple mobile phase composition and appropriate choice of chromatographic column that will achieve successful and effective, rapid and reproducible separations. We started with the idea for replacement of the acetate buffer with other less UV absorbing, with lower UV cut-off values, for better spectral analysis. We also considered omitting the presence of toxic, expensive and unstable peroxide-forming tetrahydrofuran.
The use of formiate buffer (20mm), which has UV cut-off value of about 225nm was tested and compared with acetate buffer with cut-off value of about 235nm at concentration of 20mm. These UV cut-off values are increasing with increase of buffer salt concentrations. EP, current eddtion, uses acetate buffer (50mm) which shows absorbance up to 240nm. We did not neglect their pKa values and buffering capacity, but use of mobile phase out of pH range 4.0-4.2 always resulted in decreased resolution between atorvastatin impurity B, atorvastatin and atorvastatin impurity C. Use of phosphate buffer at concentration about 10-15mm and pH 4.1±0.1 always generated best result from the resolution point, baseline flatness and faster equilibration. UV cut-off of phosphate buffers is very low, usually about 200-205nm even at high concentrations of 50mm.20
Other very interesting fact we noticed, was the identical elution sequential pattern, i.e. order of appearance of peaks with the EP described, when two totally different mobile phases were used: the original EP prescribed mobile phase, composed of acetonitrile, tetrahydrofuran and acetate buffer with pH 5.0 at different proportions during linear gradient elution method (Figure 2), and our with simple mobile phase composed of potassium phosphate buffer and acetonitrile in single stepwise gradient elution mode (Figure 3). The identical sequence of peaks was confirmed with DAD UV spectral analysis and spiking of samples and standards with single standard substance of each specified impurity, sequentially.
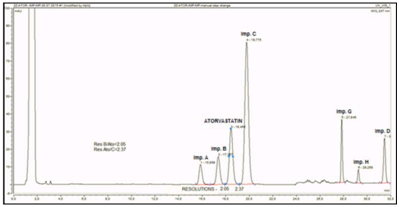
Figure 3 Separation profile of atorvastatin reference solution (c) (EP 8), additionally spiked with atorvastatin impurity C, using YMC C18 Triart 150mm x 4.6mm, 3µm, on UPLC Dionex Ultimate 3000. Critical pair resolutions: atorvastatin impurity B/atorvastatin=2.05; atorvastatin/atorvastatin impurity C=2.37.
The best results in achievement of successful and rapid, reproducible separation of atorvastatin and its impurities were obtained using YMC C18 Triart column with dimensions 150mm x 4.6mm, 3µm particles (YMC America, Inc.), with number of theoretical plates (NTP) of about 17500 regarding the main peak of atorvastatin (Figure 3). This column achieved the best separation with highest values for critical pair resolutions, for shortest run time analysis, with about 30 % lower backpressure compared with other columns with identical dimensions and particle size. The equation N=3500 x L/dp was used for calculation20
With starting mobile phase composition of 43 % v/v acetonitrile and 57 % v/v of KH2PO4 (12mm, pH 4.1) and stepwise increment for 20 % of acetonitrile (total 63 %) at 1 minute behind the 4th peak of atorvastatin impurity C, the lowest value of critical pair resolution obtained was ranging between 1.96-2.11 between atorvastatin and atorvastatin impurity B. This column showed skewed peak shapes and worsened resolution when formiate buffer (20mm, pH 4.2) was used. Mobile phase flow rate is 1.3 ml/min, detection wavelength is 244nm, and the analysis is performed at 30°C.
This stepwise increment of the percentage of acetonitrile in the mobile phase after 1 minute behind baseline touch of 4th eluted peak in the chromatogram and its return to initial percentage at 2-5 minutes (depending on type of column dimension) after the last peak of atorvastatin impurity D was our primary method development strategy during all research conducted for this article. Because of the very long retention times and capacity factors, higher than 9, for the first 4 peaks in the isocratic part of elution, change of acetonitrile presence in this isocratic elution mode for only 1% (absolute) results in significant increase of retention times. Change in retention times diminishes the critical pair resolution in both cases: higher and lower than the optimal recommended 43% v/v acetonitrile percentage.
This column was successfully tested with low dispersion UPLC and standard dispersion HPLC systems, almost with identical superiority (Figure 3-5).
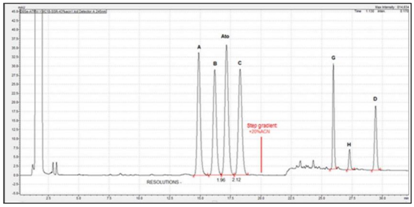
Figure 4 Separation profile of atorvastatin reference solution (c) (EP 8) using YMC C18 Triart 150mm x 4.6mm, 3µm, on UPLC Shimadzu Nexera. Critical pair resolutions: atorvastatin impurity B/atorvastatin=1.96; atorvastatin/atorvastatin impurity C=2.12.
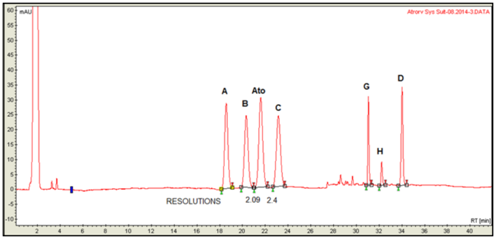
Figure 5 Separation profile of atorvastatin reference solution (c) (EP 8) using YMC 18 Triart 150mm x 4.6mm, 3µm, on HPLC Varian ProStar. Critical pair resolutions: atorvastatin impurity B/atorvastatin=2.09; atorvastatin/atorvastatin impurity C=2.40.
Chromatogram of test solution prepared from atorvastatin substance with expired shelf-life is presented in Figure 6. Some of the specified atorvastatin impurities were detected in this chromatogram, and are suitably labeled. Their identity was confirmed with comparison of the UV spectral properties of the peaks detected in the test solution with the corresponding peaks with the same RT, with known identity, originating from the atorvastatin reference solution (c) (prepared in accordance to EP 8). The other integrated but not labeled peaks in the presented chromatogram originate from other, unspecified and unidentified impurities of the active substance.

Figure 6 Chromatogram from analysis of outdated substance atorvastatin using YMC 18 Triart 150mm x 4.6mm, 3µm, on UPLC Dionex Ultimate.
It was proceeded with further investigation of this mobile phase applicability to other chromatographic columns differing in sizes, column particles and vendors. Amongst the bunch of tested columns, four of them occupied our attention in their separating power and selectivity (Table 1).
HPLC column: |
Resolution: atorvastatin impurity B/atorvastatin |
Resolution: atorvastatin/atorvastatin impurity C |
Run time (minutes): |
Pressure (bar): |
YMC 18 Triart 150 mm x 4.6mm, 3µm |
1.96 |
2.12 |
35-40 |
157 |
Nucleodur C18ec 100-3-250mm x 4.6mm, 3µm |
2.20 |
1.77 |
35-40 |
255 |
Waters Symmetry C18 250mm x 4.6mm, 5µm |
1.50 |
1.50 |
40-45 |
145 |
Superspher C18e 125mm x 4mm, 4µm |
1.41 |
1.58 |
25-30 |
131 |
Table 1 Review of some of the basic chromatographic parameters obtained with finally selected four HPLC columns that gave best results from the aspect of separating power and selectivity
Concerning the dimensions of the columns with best results, we can divide them in 3 types, for the aim of testing applicability of the proposed composition of mobile phase:
Next chromatographic separation results which will be presented are made with second most effective column Nucleodur 100-3-C18ec, 250mm x 4.6mm with 3µm particles (Figure 7). Such effective column is working with KH2PO4 buffer (12mm, pH 4.1) and higher percentage of acetonitrile (46% v/v), because of the longer length and separation power of this column, which resulted in relatively short retention times and run times. This column showed higher efficiency, normally on expanse of pressure and run time.
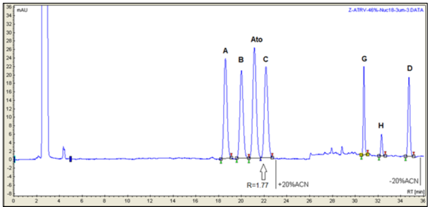
Figure 7 Separation profile of atorvastatin reference solution (c) (EP 8) using Nucleodur C18ec 250mm x 4.6mm, 3µm, on Varian LC 920. Critical pair resolutions: atorvastatin impurity B/atorvastatin=2.07; atorvastatin/atorvastatin impurity C=1.77.
Another interesting separation was achieved with Watters Symmetry C18 column, 250mm x 4.6mm, 5µm (Figure 8). The mobile phase and the rest of the chromatographic system parameters are the same as the primarly proposed ones.
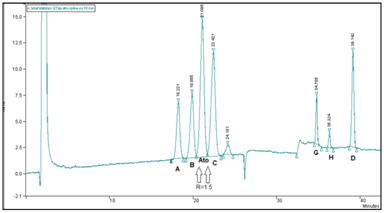
Figure 8 Separation profile of atorvastatin reference solution (c) (EP 8) using symmetry C18 250mm x 4.6mm, 5µm, on HPLC Varian ProStar. Critical pair resolutions: atorvastatin impurity B/atorvastatin=1.50; atorvastatin/atorvastatin impurity=1.50.
The picture bellow illustrates UV Spectral analysis of the obtained peaks in the chromatogram presented in Figure 8 & Figure 9. As expected, the first group of 4 peaks have almost identical absorption spectrums with high UV-wavelength absorbance ratio A244nm/A225nm values, caused by their very similar structure of isomeric-like molecules, with easily visible absorption gap around 225nm. The last 3 peaks have quite different UV absorption spectums, with lower value of UV-wavelength absorbance which are more similar between themselves when compared with any of the first 4 earlier eluting peaks. Becouse of the very high UV spectral similarities between the peaks constituting each group of peaks, lower UV-wavelegth absorbance ratio A244nm/A225nm with no pressence of “gap’ at 225nm spectrum, we performed aditional identification with comparing RRT, peak area and hight relationship with the referent chromatogram form EDQM, followed with peak spiking with each available specified impurity.
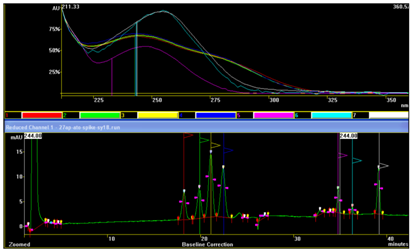
Figure 9 UV-DAD spectral investigation of peak identities of the chromatogram presented in Figure 8.
The last column dimension test strategy was performed with Supersper C18e 125mm x 4mm with 4µm particles (Figure 10-12). The mobile phase contained 42% v/v ACN in the isocratic elution mode and the rest of the chromatographic system parameters are the same as the primarly proposed ones.
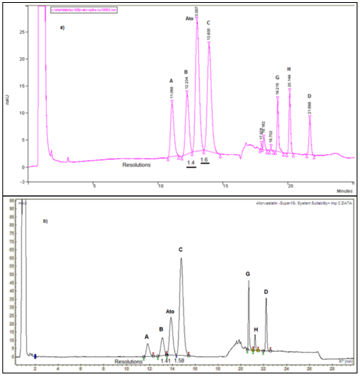
Figure 10 Separation profile of atorvastatin reference solution (c) (EP 8) using superspher C18e 125mm x 4mm, 4µm, and standard high dwell volume HPLC systems: a) HPLC Varian ProStar, b) HPLC Varian LC 920, spiked with additional quantity of atorvastatin impurity C.
The characteristic UV-spectrums of the groups with different A244nm/A225nm values and the presence (in the first group of 4 peaks) and absence (in the peaks of second group of 3 peaks) of absorption “gap region” at 225nm is again clearly visible in Figure 11. As can be seen from the chromatograms gained with standard high dwell volume HPLC systems and with more sophisticated low dwell volume - low dispersion systems, this column can be used at the edge of the permitted resolution between adjacent peaks with critical resolutions (Figure 10-12). If we use tangential peak integration we can approach up to resolutions of 1.46. Change in the mobile phase for 1% or 2% (absolute) induces significant increase in the run times of peaks, and change of pairs of peaks with critical resolutions (Figure 13). This column we recommend for use preferably with UPLC low dispersion (low dwell volume) systems, where achievement of critical resolution up to needed 1.5 is more expected.
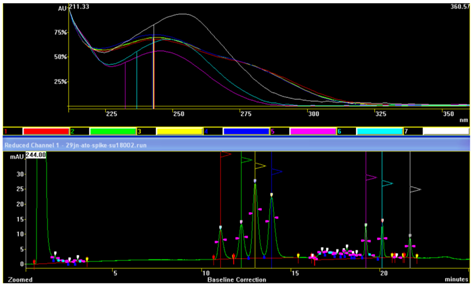
Figure 11 UV-DAD spectral investigation of peak identities of the chromatogram presented in Figure 9 a).
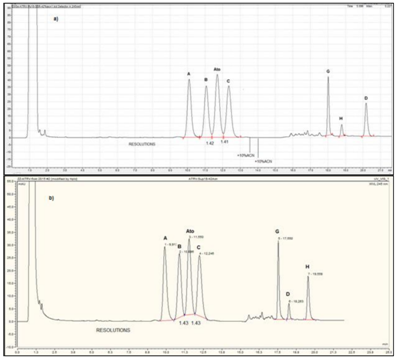
Figure 12 Separation profile of atorvastatin reference solution (c) (EP 8) using superspher C18e 125mm x 4mm, 4µm, on UPLC low dispersion (low dwell volume) systems: a) UPLC Shimadzu Nexera, b) UPLC Dionex Ultimate.
The chromatograms illustrated on Figure 13 show high dependence of peak resolutions of the percentage of acetonitrile. Changes in critical pairs of resolution and even of the identity of components which are becoming critical peaks pair can be clearly noticed. From all above presented can be seen that critical pairs of peaks, in some cases consist of atorvastatin impurity B/atorvastatin, in other of atorvastatin/atorvastatin impurity C, while in some cases of atorvastatin impurity A/atorvastatin impurity B. These could be expected due to the very similar chemical structure of the first four peaks, atorvastatin and its 3 stereoisomers. Change in the percentage of acetonitrile in mobile phase in aim to improve resolution between two targeted peaks, diminishes, i.e. reduces the resolution between other tandems of neighboring peaks. With several experimental trials the optimal percentage of acetonitrile in mobile phase can be estimated, for each individual HPLC column. The EP official method mobile phase composition confirms our conclusion, using 12% v/v tetrahydrofuran, 21% v/v acetonitrile and 67% v/v acetate buffer (50mM).
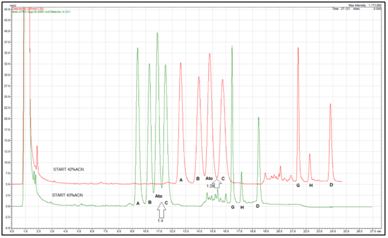
Figure 13 Illustration of influence of acetonitrile content in the isocratic part of elution on resolution, using Superspher C18e, 125mm x 4mm, 4µm column, on UPLC Shimadzu Nexera.
Pharmacopoeia recommended column for testing related substances of atorvastatin API, Zorbax Rx C8 250mm x 4.6mm, 5µm, was also tested under the conditions of this new developed method, described previously in the text. The optimal content of ACN in the isocratic part of elution was 44% v/v. Chromatograms are presented in Figure 14. Obtained critical pair resolution factors are satisfactory, i.e. all have value of ~1.5 or higher. Further research led to the interesting conclusion that using the simpler mobile phase we propose, better results were achieved with C18 (L1) bonded reversed phase columns (Figure 15), rather than with pharmacopoeia recommended C8 (L7) octylsilane columns (Figure 16).
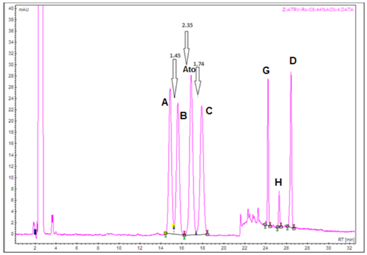
Figure 14 Testing of EP 8 recommended HPLC column, Zorbax Rx C8 250mm x 4.6mm, 5µm, with our newly developed and optimized step gradient mode of elution.
With totally different systematic approach of mobile phase composition, which was successfully applied on properly selected adequate chromatographic columns, a new simple, rapid, reproducible, and sensitive method for determination of atorvastatin impurities as well as quantification of atorvastatin itself, was created. Very important advantage of the method developed is the dual gain - with one single run, simultaneous determination of quantity of atorvastatin and its impurities can be performed. This is due to the almost perfect peak symmetry not only of small peaks of impurities but also of atorvastatin itself, as result of low injection volume of sample (not more than 7µl) dissolved in 100% dimethylformamide, appropriate mobile phase composition, and the high quality of column chromatographic silica bonded phase matrix.
The method uses single stepwise gradient elution with mobile phase without toxic and unstable tetrahydrofuran, no highly absorbing buffer components with UV cut-off values almost at or higher than the UV maximum absorbance of the analyte. It successfully separates all specified impurities of atorvastatin, achieving all needed system suitability requirements of the method in almost 3 times shorter run time than the pharmacopoeial method, depending on the choice of the used column from the group of 3 most superior columns: YMC C18 Triart 150mm x 4.6mm, 3µm; Nucledodur 250mm x 4.6mm, 3µm; and Waters Symmetry C18 250mm x 4.6mm, 5µm. The fourth shortest tested column with the smallest number of theoretical plates, NTP=12500, could achieve critical peak resolution at the “edge” of the required parameters, which lead us to conclusion that this NTP is the lowest limit for separation of atorvastatin related substance molecules, for reversed phase column type. Use of an end-capped column in this research shown to be preferable. Proposed mobile phase composition and method conditions are applicable on many different generations of HPLC and UPLC systems, without almost any impact of the type of the vendor.
Our mobile phase concept with single step gradient elution, showed favorable application of C18 octadecylsilane column chromatographic matrixes compared to C8 octylsilane based columns. Our method concept confirmed equal usability on different “generations”, classes and vendors of chromatography systems with quite different capabilities, and is prone to further improvement in order to achieve saving of time and resources.
None.
The author declares that there is no conflict of interest

©2018 Piponski, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.