Journal of
eISSN: 2473-0831


Research Article Volume 8 Issue 3
Department of Pure and Industrial Chemistry, Umaru Musa Yar’adua University Katsina, Nigeria
Correspondence: Samaila Muazu Batagarawa, Department of Pure and Industrial Chemistry, Umaru Musa Yar’adua University Katsina, Nigeria
Received: April 14, 2019 | Published: May 24, 2019
Citation: Batagarawa SM, Ajibola AK. Comparative evaluation for the adsorption of toxic heavy metals on to millet, corn and rice husks as adsorbents.J Anal Pharm Res.2019;8(3):119-125. DOI: 10.15406/japlr.2019.08.00325
This study was carried out to evaluate the efficiency of metals (Ni, Cr, and Mn) removal using, raw and carbonized rice, millet and corn husks as low cost adsorbents. The sorption of these metal ions from aqueous solution was determined using Atomic Absorption Spectroscopy Analysis (AAS). Characterisation of the adsorbents using Fourier Transform Infra Red) FTIR) and Scan Electron Microscopy (SEM) images revealed some changes before and after the adsorption process. This indicated that, most likely adsorption has been taken place between the metal ions and the adsorbents in the aqueous solution. Batch experiments were carried out to determine the effect of different parameters such as adsorbent dose, pH of the medium, contact time and agitation rate. The result obtained showed that the sorption process was largely dependent on metal ion concentration, adsorbent dose and contact time. The experimental data was analyzed using paired sample – T test. The study also showed that, all the husks were effective for the quantitative removal of various toxic heavy metals and can be used as low cost and highly efficient adsorbents for the removal of heavy metal ions from industrial effluents.
Keywords: corn husk, millet husk, rice husk, batch adsorption, toxic heavy metals, carbonised
Recently, a great deal of interest in the research for the removal of heavy metals from industrial effluents has focused on the use of agricultural by-products as adsorbents. The use of agricultural by products in bio-remediation of heavy metal ions, which is known as bio-sorption, is an aspect of Bio-technology. This is recognized as an emerging technique for the treatment of heavy metal polluted streams.1 The bio-sorption offers an alternative to remediation of industrial effluents as well as the recovery of metals contained in other media.2 According to World Health Organization3 the metals of most immediate concern are Cr III, Cr (VI), Zn, Cd, Cu. Ni, Hg, Pb, Al, Mn.4 Conventional methods for heavy metals removal from industrial waste water include reverse osmosis, electromechanical reduction, chemical precipitation, ion exchange,5 membrane filtration and co-precipitation.6 Most of these methods suffer from drawn backs as high capital and operational cost which are not suitable for small scale industries.7,8 The adsorption capacity of agricultural waste biomass varies significantly. Factors that influence the removal of heavy metals by agricultural waste biomass include types of crop residues, elements of heavy metals, pre-treatment of agricultural waste biomass and operating conditions.9 An important characteristics of good adsorbents is their high porosity and consequent large surface area with more specific adsorption, it also increases the adsorption kinetic. A better adsorbent is the one with large surface area which requires less time for adsorption. About 40 % of the weight of the harvested millet is removed as husk from the stalk. The husk is sometimes used as landfills or animal feeds. It is also found as heaps of unused wastes which are sometimes burn to ashes, thus giving rise to environmental pollution.10 The Northern part of the country is known for the large scale farming of rice, corn and millet. The husk of these materials can be used as adsorbents if processed instead of causing environmental pollution if using otherwise or not making use of it at all.
Rudre et al.,11 performed an experiment using millet husk as an adsorbent to remove cu (II) from electroplating effluent. Batch experiment was conducted at temperature 28˚C to determine parameters such as contact time, adsorbent dosage, pH and initial concentration. The maximum removal efficiency of cu(II) was 86.86 % for 1.0g/50 ml of millet husk at pH 2.0 and equilibrium contact time of 4 hours with the initial concentration of 6.24mg/l. It was concluded that millet husk has high potential adsorption of Cu(II) removal. The purpose of the research is to assess the potentials of using rice husk, millet husk and maize cob as adsorbents to remove some toxic heavy metals from aqueous solution.
Sample collection
The rice husk was collected from Funtua rice mill, while the corn and millet husks were collected at a local mill in Batagarawa Local Government, Katsina State. All the samples were collected in a polythene bag, labelled appropriately and brought to the laboratory for further analysis.
Sample treatment
The samples were washed with tap water several times to remove adhering particles. They were dried in the air for 24 hours and soaked in 1M dilute HCl at room temperature. They were filtered and washed several times with tap water and finally with distilled water until fairly constant pH were obtained. The samples were dried in the oven at 100 °C for 24 hours. The dried husks were treated according to the methods.12-14
Each of the three husks was divided into two portions. The first portion was carbonized in the murfle furnace at 500°C for 6 hours, while the second portion was grinded with pestle and mortar and sieved using 300μm sieve and stored in plastic container. The samples were labeled as Carbonized Corn Husk (CCH), Uncarbonized Corn Husk (UCH), Carbonized Millet Husk (CMH), Uncarbonized Millet Husk (UMH), Carbonized Rice Husk (CRH) and Uncarbonized Rice Husk (URH).
Batch adsorption experiments
Adsorption experiments were carried out using batch adsorption method. Various parameters such as metal ion concentrations, contact time, adsorbent dosage and pH of the solution were studied at constant agitation rate of 200 rpm, and room temperature of 25°C for each adsorption experiment. 50ml aqueous solution of each metal ion was equilibrated with varying sorbent dosage (0.2–0.6g), contact time (20-40min). The pH was adjusted to 2–10 and agitation rate of (100–300rpm). The experiments were performed using on Orbital Shaker (Scigenics Biotech Orbitek). The results were calculated based on the formular;
Where Co and Ce are the concentrations (mg/L) of metal Ions initially and at equilibrium time W is the weight of the adsorbed (mg), qe is the amount of metal ion adsorbed (mg/g), while V is the volume of the solution in litre.15-17
Reusability of the adsorbents
The reusability was done by making use of the used adsorbents, they were soaked in 50ml 0.1M HCl for 2hrs. It was filtered and rinsed in deionised water several times to remove the excess acid. It was reused by treating it with 50ml of the aqueous solution for a predetermined time and agitated rate. The filtered sample was analyzed using AAS. The reusability experiment was carried out on each of the adsorbents for the second time and the filtrate was analyzed using AAS. The procedure used was similar to that of Batagarawa and Lawal.18
Determination of specific surface area
The specific surface of the Adsorbents was estimated using Sear’s method,19 by agitating 1.5g of the adsorbent sample for each in 100ml of diluted hydrochloric acid of pH=3. Then 30g of Sodium Chloride was added with stirring and the volume was made up of 150ml with deionized water. The solution was titrated with 0.1M NaOH and the volume, v needed to raise the pH from 4–9 was then recorded. The surface area according to this method was calculated using the following equation.
Scanning Electron Microscopy (SEM)
The Scanning Electron Microscopy (SEM) experiment was carried out on the adsorbent using Philip XL 30 at accelerating voltage of 10 KV, beam size 3.0 and of different magnifications. The micrographs before and after the adsorption of metal ions and compared to study the adsorption efficiency of the adsorbent.
Statistical analysis
The analysis of the data was done using paired sample T-test. This was done to compare the means of the samples before and after adsorption. The data was presented using tables and bar charts. The statistical softwares used were Microsoft excel and SPSS.
Effect of metal ion concentration
The effects of initial metal ion concentration on the adsorption efficiency of the adsorbents are shown in (Figures 1A–C). The result shows that as the initial metal ion concentration for chromium increases, the percentage removal efficiency increased for the six adsorbents (Figure 1A). The percentage removal follows the order; 99.6% for CRH>99.5% for URH>98.9% for CCH>98.7% for CMH 98.6% for UCH and 98.5% for UMH. The percentage removal of nickel ions from aqueous solution using the six adsorbents attained equilibrium at 1mg/L is shown in Figure 1B. The optimum removal efficiency for Nickel follows the oder; 98.9% for CRH>98.8 % for CMH>98.7% URH>98.5% for CCH>97.5% for UMH and 97.0% for UCH.
Similar trend as above was observed in the adsorption of Mn(II) ions as shown in Figure 1C; with 99.3 % for CRH and 98.5 % for URH both for Rice husks. The result is in accordance with the report of Okeimen and Onyekpa.20
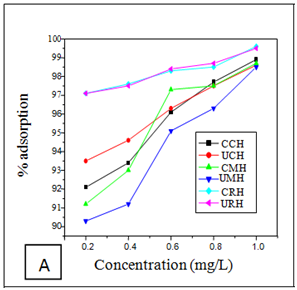
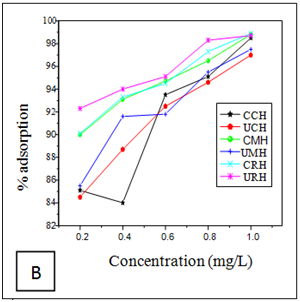
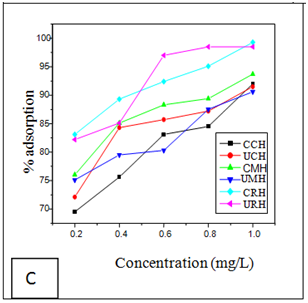
Figure 1 Effect of Metal ion Concentration on Removal of (A) Chromiun (B) Nickel and (C) Manganese removal.
Effect of adsorbent dosage
The availability and accessibility of adsorption site is controlled by adsorbent dosage.21 The effect of mass of adsorbent loading on heavy metal removal were investigated by varying adsorbent loading from 0.2 to 0.8 g per 50ml of mixed metal Ion solutions. From (Figures 2A–C), it can easily be inferred that the percentage removal of metal ions increased with increasing weight of adsorbent. This could be due to the greater availability of the exchangeable sites or surface area at higher dose.
The removal efficiency for chromium varies for CCH from 96.5% to 98.8% for UCH 98.0% to 99.0%, CMH from 95.8% to 98.1%, UMH from 83.1% to 94.7%, CRH from 93.3% to 99.5% and URH from 92.1% to 99.8%. For manganese ion, the removal efficiency varies for CCH from 91.0% to 97.6%, for UCH from 92.1% to 97.9%, for CMH from 90.0% to 98.1% for UMH from 95.1% to 98.5%, for CRH from 94.0% to 99.5% and for URH from 93.5% to 99.3%.
Effect of agitation rate
The effect of agitation rate was studied by using the agitation rate of 100 rpm, 200 rpm and 300 rpm respectively, keeping all other factors constant, that is using concentration of 1.0mg/l and contact time of 60min for the adsorption of chromium, Nickel ion and Manganese using six different adsorbents. At 200rpm, the adsorption of the metal reached maximum for all the three metals using CCH, UCH, CMH, UMH, CRH and URH as adsorbents as shown in (Figures 3A–C). This is due to the attractive between the metal and adsorption site of the Adsorbents. At the higher agitation rate, the molecule of the metals do not have enough time for contact with the active sites of the adsorbents, because the rate of agitation is very fast. Increasing the agitation rate at moderate level enhance the metal ion diffusion to the surface of the adsorbents and also causes adsorption in the film boundary layer around the adsorbents, thereby leading to an increase in adsorption. Decrease in percentage of metal ions adsorbed as the rate of agitation increase could be as a result of desorption of some metal Ions from the surface of the adsorbent due to too vigorous agitation rate.
Effect of contact time
The effect of contact time on the adsorption efficiency was shown in (Figures 4A–C). Adsorption experiments were carried out at different time intervals; 20, 30 and 40 min in mixed metal Ions. It was observed that the removal of tested metal was rapidly achieved within a short period of 20min. Adsorption of chromium and nickel Ions attained maximum within 20min while that of manganese within 30min. previous results by some researchers revealed that removal of all tested metal was rapidly removed within a period of 30min.22 The effect of contact time on adsorption process of metal Ions from waste waters were studied by many authors.23-26 The result indicated that the equilibrium time was dependent on the nature of the adsorbent and on metal Ions concentration.
Effect of pH
The pH adsorption for Cr, Ni and Mn are shown in (Figures 5A–C) respectively. All the experiments were carried out in the pH values of 2, 6 and 10, where chemical precipitation is almost avoided, so that metal removal could be related to the adsorption process.27 The susceptibility of the system pH changes may be attributed to the nature of the ions in solution and the nature of the adsorbent used. The lower the pH, the more the H+ ions competing with the metal ions for adsorption sites, thus reduces their adsorption. On the other hand, the higher the pH the less the H+ ions competing with metal ions for adsorption sites, thus increasing their adsorption.
The maximum adsorption of Ni and Mn occurred at pH 6.0, which was similar to the result obtained for the adsorption of Cd(II) ions by coconut copra meal.28,29
Chromium ions are available as CrO32- or HCrO3- in the solution. It has been found that bio sorption of chromium decreases with increases in the pH of the solution. Possible reason may be the presence of more number of sites on the bio sorbent at lower pH which can absorb chromate ions. At high pH, precipitation usually occurred between metal ions and hydroxide ions.28
Surface area analysis
The Sear’s Specific Surface Area of the adsorbents samples is presented in Table 1. It has been reported that a single gram of adulterated carbon can have a surface area in excess of 500(m2g-1), with 1500m2g-1 being readily achievable. Therefore CCH, UCH, CMH, UMH, CRH and URH having specific surface area of 524m2g-1, 519m2g-1, 527m2g-1, 520m2g-1, 537m2g-1 and 531m2g-1 respectively and therefore were in agreement with the literature. And we can see from the table that all the adsorbents have good surface area that allowed them adsorbed effectively, but CRH, with highest surface area adsorbed more than others.
|
CCH |
UCH |
CMH |
UMH |
CRH |
URH |
Sear’s specific surface area (m2g-1) |
524 |
519 |
527 |
520 |
537 |
531 |
Table 1 The surface area of the various adsorbents
Fourier Transform Infrared (FT-IR) Spectroscopy
FTIR technique is an important tool to identify some characteristics functional groups, which are capable of adsorbing metal ions and at the same time instrumental in adsorption process. The most important constituent of agricultural waste are carbohydrates, therefore the functional groups of choice are C=O and OH (Figure 6).
The FT IR graph showed a strong intensity band at 3460cm-1, which is associated with OH from water and other carbohydrates present. The spectra also shows a band at 1637cm-1, which is attributed to the presence of Carbonyl (C=O) from cellulose, in addition to a strong absorption at 1075cm-1 which is due to Si-O-Si vibration.
Scanning Electron Microscopy (SEM)
Scanning Election Microscopy (SEM) has been extensively used to characterize the microstructure of carbonized corn husks before and after adsorption. The morphological study of the micrographs for carbonised and uncarbonised husks is shown in plates 1 and 2 respectively. The SEM in plate 1 showed partially developed honey comb-like and highly defined pores. It shows that, carbonization influenced the topographical characteristics of the adsorbents. Plate 2 shows the surface structure after adsorption of Nickel metal from aqueous solution. The image revealed that the external surface consist of small cavities, characterized by irregular heterogeneous surface which suggested presence of adsorbed metal ions on the surface. Before metal uptake for both carbonized and uncarbonized corn husks, the images revealed that the external surface was full of cavities, roughly characterized by irregular heterogeneous surface which suggested that, uncarbonised corn husk exhibit high surface area, however, the carbonized corn husk has more distinguish pores than that of uncarbonized corn cob, indicating that it will have more surface area than uncarbonized corn cob, and large surface area increased the rate of adsorption. Absence of some pores and lighter surface of the adsorbents after metal ion adsorption suggested that, metal ions adsorption had taken place (Figure 7).
Statistical analysis
To have an insight into the effectiveness of CCH, UCH, CMH, UMH, CRH and URH in the removal of heavy metal ions from aqueous solutions, paired sample t-test analysis was carried out on the results. Paired sample t-test analysis was used to find out the level of significance between two values. The initial concentration of the metal ions in the aqueous solution was compared to the final concentration after metal ion adsorption to see if there is a level of significance in the amount of metal ion adsorbent. The significance level determines the effectiveness of the adsorbents in the removal of heavy metals from waste water sample. The adsorbents are effective in the removal of heavy metals from waste samples if the level of significance is less than 0.05.
The removal of chromium ions gave the following level of significance; 0.013, 0.014, 0.013, 0.014 and 0.014, while Nickel ions have the following values; 0.014, 0.014, 0.014, 0.014, 0.016 and 0.013. Manganese ions showed the following level significance of removal; 0.014, 0.014, 0.014, 0.014, 0.014, and 0.021. From the results obtained, it can be observed that, significant level of less than 0.05 was obtained for all the four adsorbents. It can therefore be concluded that the adsorbents were very effective in the removal of Chromium, Nickel and Manganese from aqueous solutions.
This study showed that the three adsorbents used (Rice husk, millet husk and corn husk) are promising adsorbents for the removal of nickel, chromium and manganese ions from aqueous solution. Initial metal ion concentration, adsorbent dosage, contact time, rate of agitation and pH as factors that affect adsorption process of metal were studied using aqueous solution. The maximum uptake of all the three metals occurred at constant time of 20min., agitation rate of 200rpm, adsorbent dosage of 0.2g and metal ion concentration of 0.6mg/l. Pair sample test was used to determine the efficiency of the biosorbents on the removal of the Chromium, Nickel and Manganese. It can be observed that, all the three adsorbents were good for the removal of heavy metal ions in aqueous solution. The development of these adsorbents for the treatment of effluents from Industries and domestic wastes is therefore recommended. This will reduce the harmful effect of residual metal ions found in ground water, soil-sediments water ways and many other sources.
None.
The authors declare that there is no conflict of interests.

©2019 Batagarawa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.