Journal of
eISSN: 2473-0831


Research Article Volume 6 Issue 1
Correspondence: Fouda AS, Department of Chemistry, Faculty of Science, El-Mansoura University, El-Mansoura- 35516, Egypt, Tel +2 050 2365730, Fax +2 050 2202271
Received: August 26, 2017 | Published: September 18, 2017
Citation: Fouda AS, Emam A, Refat R, Nageeb M (2017) Cascabela Thevetia Plant Extract as Corrosion Inhibitor for Carbon Steel in Polluted Sodium Chloride Solution. J Anal Pharm Res 6(1): 00168. DOI: 10.15406/japlr.2017.06.00168
The inhibiting effect of Cascabela Thevetia extract (CTE) in polluted sodium chloride (3.5% NaCl+16 ppmNa2S) on the corrosion of carbon steel (CS) was studied by weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS) techniques. The results showed that the inhibition efficiency (IE) increases with increase in extract dose and decreases with raising the temperature. %IE reached to ≈95% at 300 ppm CTE. The obtained results indicated that the investigated extract is physically adsorbed on the CS surface and follows Freundlich adsorption isotherm. The PP data revealed that this extract acts as mixed type inhibitor. Surface morphology was tested using atomic force microscope (AFM) technique. Some quantum chemical calculations were used to support the experimental data. The results obtained from the different techniques are in good agreement.
Keywords: corrosion inhibition, carbon steel, polluted nacl, cascabela thevetia extract, adsorption
CTE, cascabela thevetia extract; CS, carbon steel; WL, weight loss; PP, potentiodynamic polarization; EFM, electrochemical frequency modulation; EIS, electrochemical impedance spectroscopy; IE, inhibition efficiency; AFM, atomic force microscope; SCE, saturated calomel electrode; OCP, open circuit potential
Corrosion of materials causes big losses in the economy of many countries due to the huge amount of funds needed to reduce it. Corrosion inhibition of metals, especially of CS, has received some attention because of its excellent mechanical properties, low cost and widespread use in the industry such as chemical processing, petroleum production, refining, transportation, pipelines, mining, construction industry, marine applications, thus, researches in the field of corrosion inhibition of steel is necessary. Seawater contains the remains of dead organisms, coral reefs, ships and boats which all are rot and lead to escalation of gases such as H2S and Na2S and other gases react with chlorine and hydrogen produce polluted NaCl. Among the several methods of corrosion control is the use of inhibitors. The studies of plant extracts as corrosion inhibitors are of great interest from an environmental perspective and are attracting a significant level of attention.1-3 The safety and environmental issues of corrosion inhibitors arisen in industries has always been a global concern. Green inhibitors which have a promising future for the quality of the environment because they do not contain toxic compounds or heavy metals as Pb or Fe, so in the marine environment we should use natural plant inhibitors to overcome the corrosion problem and to protect the marine environment at the same time. So receive attention for the replacement of synthesized inorganic inhibitors which are often toxic, expensive, carcinogenic and environmentally unfriendly.4 Plant extract is low cost and environmental safe, so the main advantages of using plant extracts as corrosion inhibitors are economic and safe environment. Several studies have been carried out on the protection of corrosion of metals and alloys by plant extracts such as: coffee ground,5 Ruta Graveolens,6 Murraya Koenigii,7 Watermelons Rind,8 Oxandra Asbeckii,9 Euphorbia Falcata,10 Osmanthus Fragran Leaves,11 Punica Plant,12 Henna,13 Tita Cordata,14 Curcum plant15 Delonix Regia leaf.16 Nonetheless, there is still a need for research on other plants can be used as inhibitors in industrial applications.
This present study seeks to:
Materials and solutions
Tests were performed on rectangular specimens with dimensions 2x2x0.1cm of CS with the following chemical composition in weight %: C 0.200, Mn 0.350, P 0.024, Si 0.003 and Fe balance. CS samples (2x2x0.2cm) mechanically abraded with different grades (22-1200) of sand paper and polished by 0.25 micron alumina powder to approach the mirror surface, then degreased, hand washed with bidistilled water and rinsed with ethanol, finally used in weight loss tests.
Solution preparation
The polluted sodium chloride (3.5%NaCl+16ppmNa2S) solution was prepared from NaCl and Na2S (Merck) and bi-distilled water. The concentration range of Cascabela Thevetia extract varied from 50 to 300ppm and the electrolyte used was 100 ml for each experiment.
Stock solution of cascabela thevetia extract
Stock solution of the Cascabela Thevetia extract was prepared by boiling 20-25g of air-dried Cascabela Thevetia adult leaves in bidistilled water and left overnight. The Cascabela Thevetia leaves were air-dried earlier in natural sunlight until the final mass became constant. The contents of the extraction process were then mixed in a plinder, filtered and the resulting solution was kept in a refrigerator at low temperatures of 2-3oC in order to prevent the contents of the extract from being altered due to the chemical, physical, and biological reactions it might otherwise underg.17
Weight loss measurements
The weighted samples they immersed in 100ml of polluted NaCl with and without different doses of CTE. The immersion time of the weight loss test is 30, 60, 90,120,150 and180min the CS samples were taken out, washed with bidistilled water, dried and weighted again. The results of the weight loss tests are the mean of three runs, each with fresh specimen and 100 ml of fresh polluted NaCl solution. The inhibition efficiency (%IE) and the degree of surface coverage (θ) were calculated using the following equation:
(1)
Where Wo and W are the values of the average weight losses in the absence and presence of the extract, respectively.
Polarization measurements
Polarization experiments were carried out in a conventional three-electrode cell with platinum gauze as the auxiliary electrode and a saturated calomel electrode (SCE) coupled to a fine Luggin capillary as reference electrode. The working electrode was in the form of a square cut from CS sheet of equal composition embedded in epoxy resin of poly tetrafluoroethylene so that the flat surface area was 1 cm2. Prior to each measurement, the electrode surface was pretreated in the same manner as the weight loss experiments. Before measurements, the electrode was immersed in solution for 30 min. until a steady state was reached. The potential was started from -600 to +400mV vs open circuit potential (OCP) with a scan rate 1 mVs-1. All experiments were performed at 25oC and the results were always repeated at least three times to check the reproducibility. The %IE and θ were calculated using the following equation:
(2)
Where, iocorr and icorr are corrosion current densities of CS in the absence and presence of extract, respectively.
Electrochemical impedance spectroscopy (EIS)
Impedance measurements were carried out in the frequency range of 100 kHz to 0.1 Hz and peak to peak ac amplitude of 5 mV. All impedance data were fitted to appropriate equivalent circuit using the Gamry Echem Analyst software version 6.03.The %IE and θ were calculated using the following equation:
(3)
Where, Roct and Rct are the charge-transfer resistance values without and with inhibitor respectively. The double layer capacity was calculated using the following equation:
(4)
Where fmax is the frequency maximum
Electrochemical frequency modulation (EFM)
EFM experiments were performed with applying potential perturbation signal with amplitude 10 mV with two sine waves of 2 and 5 Hz. The choice for the frequencies of 2 and 5Hz was based on three arguments.18 The larger peaks were used to calculate the corrosion current density (icorr), the Tafel slopes (βc and βa) and the causality factors CF-2 and CF-.19
All electrochemical experiments were carried out using Gamry instrument PCI300/4 Potentiostat/Galvanostat/Zra analyzer, DC105 corrosion software, EIS300 electrochemical impedance spectroscopy software, EFM140electrochemical frequency modulation software and Echem Analyst 5.5 for results plotting, graphing, data fitting and calculating.
Surface analysis
For morphological study, surface features (1x1x0.1cm) of CS were examined before and after exposure to polluted NaCl solutions for 24 hours with and without the CTE.
Weight Loss (WL) Measurements
WL of CS was determined, at various time intervals without and with different doses of CTE. The obtained WL- time curves are represented in Figure 1 for CTE. The IE of this extract was found to be dependent on the extract dose. The curves obtained in the presence of the extract fall significantly below that of its absence. The increase in the extract dose was accompanied by a decrease in WL, an increase of θ and an increase in the %IE. These results lead to the conclusion that this extract is fairly efficient as inhibitor for CS dissolution in polluted NaCl solution. The data obtained are summarized in Table 1.
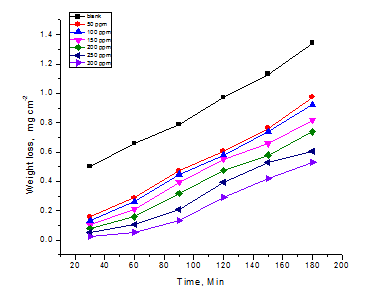
Figure 1 WL-time curves for the corrosion of CS in polluted NaCl without and with different doses of Cascabela Thevetia extract at 25ºC.
|
Conc., ppm |
CR, x10-4 mg cm2 min-1 |
θ |
% IE |
|
blank |
80 |
--- |
--- |
|
50 |
50 |
0.378 |
37.8 |
|
100 |
48 |
0.405 |
40.5 |
|
150 |
46 |
0.432 |
43.2 |
|
200 |
39 |
0.514 |
51.4 |
|
250 |
33 |
0.595 |
59.5 |
|
300 |
24 |
0.703 |
70.3 |
Table 1 Values of % IE, θ and corrosion rate (CR) of different doses of Cascabela Thevetia extract for the corrosion of CS from WL measurements at 25ºC
Effect of temperature
The effect of temperature on the CR of CS in polluted NaCl in presence of different doses of the extract was studied in the temperature range of 25-45oC using WL measurements. As indicated from Table 2, the rate of CS dissolution increases as the temperature increases, but at lower rate than in uninhibited solutions, this indicated that the raising of temperature led to the reduction of the extract adsorption and then the acceleration of dissolution process,20 which proves that the adsorption of the extract on the surface of CS occurs through physical adsorption.
Adsorption isotherms
|
Conc., ppm |
Temp., 0C |
CR, x10-4 mg cm2 min-1 |
θ |
% IE |
|
Blank |
25 |
80 |
----- |
----- |
|
30 |
85 |
----- |
----- |
|
|
35 |
90 |
----- |
------ |
|
|
40 |
105 |
------ |
------ |
|
|
45 |
110 |
------ |
------- |
|
|
50 |
25 |
50 |
0.378 |
37.8 |
|
30 |
58 |
0.306 |
30.6 |
|
|
35 |
67 |
0.253 |
25.3 |
|
|
40 |
83 |
0.227 |
22.7 |
|
|
45 |
88 |
0.217 |
21.7 |
|
|
100 |
25 |
48 |
0.405 |
40.5 |
|
30 |
53 |
0.361 |
36.1 |
|
|
35 |
60 |
0.331 |
33.1 |
|
|
40 |
73 |
0.318 |
31.8 |
|
|
45 |
78 |
0.304 |
30.4 |
|
|
150 |
25 |
46 |
0.432 |
43.2 |
|
30 |
51 |
0.389 |
38.9 |
|
|
35 |
58 |
0.356 |
35.6 |
|
|
40 |
71 |
0.341 |
34.1 |
|
|
45 |
76 |
0.326 |
32.6 |
|
|
200 |
25 |
39 |
0.514 |
51.4 |
|
30 |
44 |
0.472 |
47.2 |
|
|
35 |
51 |
0.434 |
43.4 |
|
|
40 |
63 |
0.409 |
40.9 |
|
|
45 |
68 |
0.391 |
39.1 |
|
|
250 |
25 |
33 |
0.595 |
59.5 |
|
30 |
37 |
0.556 |
55.6 |
|
|
35 |
44 |
0.511 |
51.1 |
|
|
40 |
56 |
0.477 |
47.7 |
|
|
45 |
61 |
0.457 |
45.7 |
|
|
300 |
25 |
24 |
0.703 |
70.3 |
|
30 |
30 |
0.639 |
63.9 |
|
|
35 |
35 |
0.614 |
61.4 |
|
|
40 |
44 |
0.590 |
59.0 |
|
|
45 |
47 |
0.585 |
58.5 |
Table 2 Data of WL measurements for CS in polluted NaCl solution with and without different doses of CTE at 25-450C
One of the most convenient ways of expressing adsorption quantitatively is by deriving the adsorption isotherm that characterizes the metal/inhibitor/ environment system. The basic information on the interaction between the inhibitor and the metal surface can be provided by the adsorption isotherm, and the type of the inhibitors on metal is influenced by:
The values of the (θ) were evaluated at different doses of the extract in polluted NaCl solution. Various adsorption isotherms were applied to fit θ values, but the best fit was found to obey Freundlich adsorption isotherm which is represented in Figure 2 for Cascabela Thevetia at 25ºC, Freundlich adsorption isotherm expressed by Eq. 5:
(5)
Where, C is the dose (ppm) of the extract in the bulk electrolyte, and Kads is the adsorption equilibrium constant. A plot of θ versus C should give straight lines with slope equals to 2.303/a and intercept (2.303/a log Kads). The experimental data give good curves fitting for the applied adsorption isotherm as the correlation coefficients (R2) were in the range (0.978-0.919). Kads obtained from the intercepts of Freundlich adsorption isotherm and is related to free energy of adsorption ΔGºads as follows:
(6)
Where, R is the universal gas constant, T is the absolute temperature and 55.5 is molar dose of water in the solution.
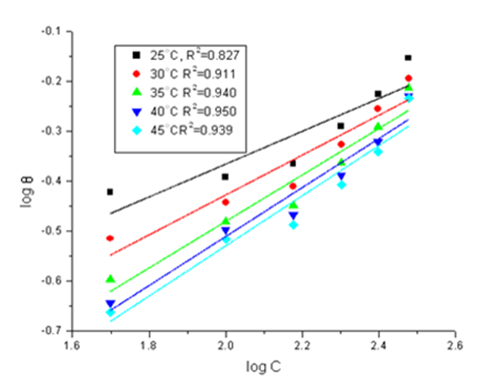
Figure 2 Freundlich adsorption isotherm for Cascabela Thevetia extract on CS surface in polluted NaCl at different temperatures.
The value of Kads and ΔGºads for the plant extract were calculated and are recorded in Table 3.The negative value of ΔGoads ensures the spontaneity of the adsorption process and stability of the adsorbed layer on the steel surface. Generally, values of ΔGoads around -20kJ mol-1 or lower are consistent with the electrostatic interaction between the charged molecules and the charged metal (physisorption). The values of Kads were found to run parallel to the %IE. The heat of adsorption (ΔHoads) and the change in entropy (ΔSoads) can calculated according to the thermodynamic basic Eq.7.
(7)
The absolute values of ΔHºads obtained in this study was lower than (40kJ mol−1), this indicative of physisorption.21 In the present case, the negative values of ΔH°ads indicate that this extract may be adsorbed physically and chemically on CS surface. The ΔSoads values in the presence of extract in polluted NaCl are negative. This indicates that decrease in disorder takes places on going from reactants to the metal-adsorbed reaction complex.22
|
Temp. K |
Kads M-1 |
-∆G°ads kJ mol-1 |
-∆H◦ads kJ mol-1 |
-∆S◦ads J mol-1K-1 |
|
298 |
0.094 |
4.1 |
46.5 |
142.2 |
|
303 |
0.059 |
3.0 |
143.5 |
|
|
308 |
0.038 |
2.0 |
144.6 |
|
|
313 |
0.032 |
1.5 |
143.7 |
|
|
318 |
0.029 |
1.3 |
142.2 |
Table 3 Thermodynamic parameters from Freundlich isotherm model for CS in polluted NaCl for investigated extract at different temperatures
Kinetic –thermodynamic corrosion parameter
The activation parameters for the corrosion process were calculated from Arrhenius-type plot according to Eq.8:
(8)
Where, E*a is the apparent activation energy, R is the universal gas constant and A is the Arrhenius pre-exponential constant. Values of apparent activation energy of corrosion for CS in polluted NaCl without and with various doses of CTE are shown in Table 4. These values were determined from the slope of log (kcorr) versus 1/T plots are shown in Figure 3. The results of Table 5 showed that the value of (E*a) for inhibited solution is higher than that for uninhibited solution, suggesting that dissolution of CS is slow in the presence of the extract. It is known from Arrhenius equation that the higher (E*a) values lead to the lower corrosion rate, this is due to the formation of a film on the CS surface serving as an energy barrier for the CS corrosion.23 Schmidt et al.24 found that organic molecules inhibit both the anodic and cathodic partial reactions on the electrode surface and a parallel reaction takes place on the covered area but the reaction rate on the covered area is substantially less than on the uncovered area similar to the present study. The alternative formulation of transition state equation is shown in Eq. 9:
(9)
Where, kcorr is the rate of metal dissolution, h is Planck’s constant; N is Avogadro’s number, is the entropy of activation and is the enthalpy of activation. Figure 4 shows a plot of (log kcorr/T) against (1/T) in the case of Cascabela Thevetia in polluted NaCl. Straight lines are obtained with a slopes equal to (ΔH* /2.303R) and intercepts are [log (R/Nh + ΔS*/2.303R)]. These results are presented in Table 4.
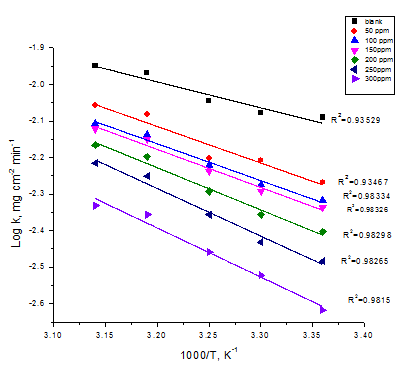
Figure 3 Log kcorr vs. 1/T curves for CS corrosion after 120 minutes of immersion in polluted NaCl with and without various doses of CTE.
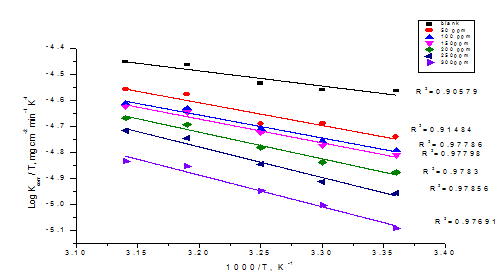
Figure 4 Log (kcorr/ T) vs 1/T curves for transition plots for CS corrosion rates (kcorr) after 120 minutes of immersion in polluted NaCl with and without various doses of CTE.
|
Comp |
Conc., ppm |
Ea*, kJ mol-1 |
∆H*, kJ mol-1 |
-∆S*, J mol-1K-1 |
|
Polluted NaCl |
0.0 |
7.0 |
5.4 |
263.1 |
|
Cascabela thevitia |
50 |
19.1 |
16.7 |
232.7 |
|
100 |
19.3 |
16.9 |
232.3 |
|
|
150 |
19.9 |
17.5 |
231.0 |
|
|
200 |
22.0 |
19.6 |
225.1 |
|
|
250 |
24.9 |
22.5 |
217.1 |
Table 4 Thermodynamic activation parameters for the dissolution of CS in polluted NaCl with and without different doses of investigated extract
|
Conc., ppm |
- Ecorr., mV vs SCE |
icorr., μAcm-2 |
βa, mV dec-1 |
βc, mV dec-1 |
kcorr, mmy-1 |
θ |
%IE |
|
Polluted NaCl |
980 |
134.0 |
95 |
100 |
171 |
-- |
-- |
|
50 |
971 |
23.6 |
98 |
81 |
92 |
0.823 |
82.3 |
|
100 |
978 |
22.0 |
103 |
72 |
71 |
0.835 |
83.5 |
|
150 |
991 |
16.1 |
106 |
90 |
67 |
0.879 |
87.0 |
|
200 |
969 |
15.7 |
89 |
85 |
60 |
0.882 |
88.2 |
|
250 |
988 |
9.6 |
95 |
79 |
58 |
0.928 |
92.8 |
|
300 |
982 |
7.4 |
100 |
99 |
57 |
0.944 |
94.4 |
Table 5 Ecorr, icorr, βc,βa, θ, and % IE of CS in polluted NaCl at 25°C for CTE
The positive signs of the enthalpies (ΔH*) reflect the endothermic nature of the CS dissolution process. The entropy of activation in presence and absence of the extract was large and negative. This implies that the activated complex in the rate determining step represents association rather than dissociation, indicating that a decrease in disorder takes place, going from reactant to the activated complex.25
Potentiodynamic Polarization (PP) Measurements
Figure 5 shows the PP curves of CS dissolution in polluted NaCl in the absence and presence of different doses of Cascabela Thevetia extract at 25ºC. The numerical values of the variation of the corrosion current density (icorr), the corrosion potential (Ecorr), Tafel slopes (βa and βc),), the (θ) and %IE were recorded in Table 5. The IE and θ were calculated using Eq.2
The results of Table 5 indicate that:

Figure 5 PP curves for the dissolution of CS in polluted NaCl with and without different doses of CTE at 25oC.
Electrochemical impedance spectroscopy (EIS) measurements
EIS is well-established and it is a powerful technique for studying the corrosion. Surface properties, electrode kinetics and mechanistic information can be obtained from impedance diagrams26 (Figure 6). Figure 7 shows the Nyquist plots obtained at open circuit potential both in the absence and presence of increasing doses of investigated CTE at 25°C. The increase in the size of the capacitive loop with the addition of investigated extract shows that a barrier gradually forms on the CS surface. The Nyquist plots do not yield perfect semicircles as expected from the theory of EIS. The deviation from ideal semicircle was generally attributed to the frequency dispersion27 as well as to the inhomogenities of the surface.

Figure 7 AFM micrograph of CS surface (a) before immersion in polluted NaCl, (b) after 24h immersion in polluted NaCl,(c) after 24h immersion in polluted NaCl + 300 ppm of CTE
EIS spectra of the investigated extract were analyzed using the equivalent circuit, Figure 6, which represents a single charge transfer reaction and fits well with our experimental results. The constant phase element, CPE, is introduced in the circuit instead of a pure double layer capacitor to give a more accurate fit.28 The double layer capacitance, Cdl, for a circuit including a CPE parameter (Y0 and n) were calculated the following Eq. 10:29
(10)
Where Y0 is the magnitude of the CPE, ω = 2πfmax, fmax is the frequency at which the imaginary component of the impedance is maximal and the factor n is an adjustable parameter that usually lies between 0.5 and 1.0. After analyzing the shape of the Nyquist plots, it is concluded that the curves approximated by a single capacitive semicircles, showing that the corrosion process was mainly charged-transfer controlled.30 The general shape of the curves is very similar for all samples (in presence or absence of extract) indicating that no change in the corrosion mechanism.31 From the impedance data (Table 6), we concluded that the value of Rct increases with increasing the dose of the extract and this indicates an increase in % IEEIS, which in concord with the PP results obtained. In fact the presence of extract enhances the value of Rct in acidic solution. Values of double layer capacitance are also brought down to the maximum extent in the presence of extract and the decrease in the values of CPE follows the order similar to that obtained for icorr in this study. The decrease in CPE/Cdl results from a decrease in local dielectric constant and/or an increase in the thickness of the double layer, suggesting that organic derivatives inhibit the iron corrosion by adsorption at metal/acid32 (Figure 8).
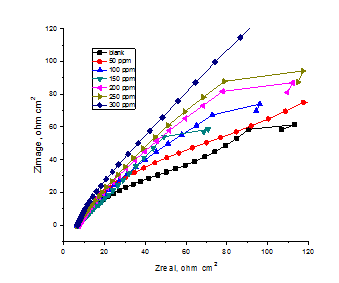
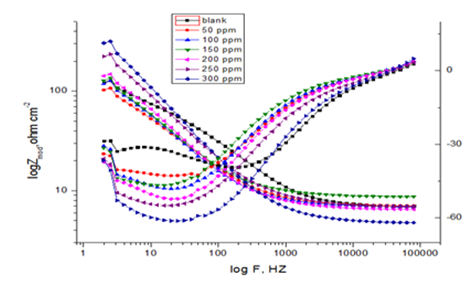
Figure 8 Nyquist (a) and Bode (b) plots for the corrosion of CS in polluted NaCl with and without different doses of CTE at 25o C.
|
Conc.,ppm |
Rct, Ω cm2 |
Cdl, Fcm-2 |
θ |
%IE |
|
Polluted NaCl |
57 |
685 |
----- |
----- |
|
50 |
95 |
665 |
0.393 |
39.3 |
|
100 |
229 |
550 |
0.747 |
74.7 |
|
150 |
258 |
522 |
0.779 |
77.9 |
|
200 |
300 |
422 |
0.810 |
81.0 |
|
250 |
434 |
230 |
0.866 |
86.6 |
|
300 |
650 |
205 |
0.912 |
91.2 |
Table 6 Electrochemical kinetic parameters obtained from EIS technique for CS in polluted NaCl with and without different doses of investigated extract
Electrochemical frequency modulation (EFM) measurements
Figure 9 shows the EFM intermodulation spectra (current vs. frequency) of CS in polluted NaCl solutions containing different concentrations of Cascabela Thevetia extract. The larger peaks were used to calculate the corrosion current density (icorr), the Tafel slopes (βc and βa) and the causality factors CF-2 andCF-3. These electrochemical parameters were listed in Table 7. The data presented obviously show that, the addition of the tested extract at a given dose to the acidic solution decreases the corrosion current density, indicating that this extract inhibits the corrosion of CS in polluted NaCl through adsorption. The causality factors obtained under different experimental conditions are approximately equal to the theoretical values (2 and 3) indicating that the measured data are verified and of good quality. The inhibition efficiencies % IEEFM increase by increasing the extract doses and was calculated from the following Eq 11.
(11)
Atomic force microscope (AFM) studies
AFM is a new technique to study the influence of extract on the generation and the progress of corrosion at the metal surface. The main advantage of this method is that the roughness of the surface can be determined. Figure 6(a) showed AFM image of CS specimen before immersion in polluted NaCl in 3D. Figure 6(b) is an image obtained for CS specimen after immersion in polluted NaCl without extracts in3D. Figure 6(c) referred to CS specimen after 24h in immersion of polluted NaCl + 300 ppm of Cascabela Thevetia extract in 3D.
As shown in Table 8, the lower value of roughness in the presence of extract as compared to its absence that the adsorption of extract molecules on the CS surface. So, it inhibits the corrosion of CS in polluted NaCl, suggested that the extract acts as good corrosion inhibitor. The lower value of roughness in the presence of Cascabela Thevetia suggested that Cascabela Thevetia protects the CS surface in polluted NaCl.
Mechanism of corrosion inhibition
Cascabela Thevetia extract used as an inhibitor leads to changes in the Tafel slopes, decrease of the corrosion current density, an increase in the charge transfer resistance and decrease in the double layer capacitance through the interference in cathodic and anodic reactions. The mechanism of inhibition is due to the physical adsorption of extract molecules on the CS surface. Most of the Cascabela Thevetia constituents are proteins, lipids, soluble sugar, starch and ash. These molecules may inhibits the corrosion due to:
From the results of the study the following may be concluded:
The investigated extract is good corrosion inhibitor for CS in polluted NaCl solution. Results obtained from potentiodynamic polarization indicated that the investigated extract is mixed-type inhibitor. % IE of Cascabela Thevetia extract was temperature dependent and its addition led to an increase of the activation energy. The results of EIS revealed that an increase in the charge transfer resistance and a decrease in double layer capacitance when the extract is added and hence an increase in % IE. This is attributed to increase of the thickness of the electrical double layer. The adsorption of Cascabela Thevetia extract onto CS surface follows the Freundlich adsorption isotherm model (physisorption). Reasonably good agreement was observed between the values obtained by the weight loss and electrochemical measurements.
None.

©2017 Fouda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.