Journal of
eISSN: 2473-0831


Research Article Volume 12 Issue 1
Institute of Science, Technology and Quality, Brazil
Correspondence: Renan Marcel Bonilha Dezena, Institute of Science, Technology and Quality, Campinas, Brazil
Received: January 03, 2023 | Published: January 10, 2023
Citation: Dezena RMB, Gomes BHP, Teixeira MB, et al. Cancer: A problem of public health in Brazil. J Anal Pharm Res. 2023;12(1):6-7. DOI: 10.15406/japlr.2023.12.00416
Cancer being one of the most important causes of death, investments in research and innovation to better understand the mechanisms of this pathology and, consequently, the development of new therapies are extremely important and necessary to benefit patients and save lives.
The process of drug development is highly costly and these costs have progressed significantly over time. The main factors that directly affect the launch of new pharmaceuticals are mainly time and risk.1
The pharmaceutical industry has invested more than $ 1.36 trillion in research and development (R&D) over the period 2007-2016. The financial resources put into research are growing at an average of 4% a year, with the most recent annual growth increasing to 6%. The annual investment is estimated to be approximately $ 204 billion by 2024.2 The global pharmaceutical industry invests around 20% of its revenue in research, development and innovation.
The modern concept employed for the development of new drugs involves: discovery of therapeutic targets; designing and selecting the leader molecule for the intended target; optimization of the leader molecule; development of the candidate; and finally, the discovery of the drug. For every 30.000 synthesized molecules, 20.000 (66.7%) enter the preclinical studies phase, 200 (0.67%) enter phase I clinical studies; 40 (0.13%) go to phase II, 12 (0.04%) enter phase III and only 9 (0.027%) are approved by regulatory bodies. It is important to mention that only one approved drug (0.003%) satisfies the market and, as a result, it brings a return to the industry that developed it.3
What is happening nowadays is that the discovery of new pharmacological targets, meaning the first stage of the process leading to the discovery of the drug, is scarce. Thus, there has been a substantial reduction in the development of new drugs in recent years.
The Brazilian drug market can be considered potentially relevant for the pharmaceutical industry, since there has been an increase of income distribution and also aging of the population, causing significant increase in this market. Despite this, the pharmaceutical industry based on Brazilian national investments is still very incipient, being considered a transformation industry. Thus, the vast majority of pharmaceutical inputs and some finished drugs are imported from major developed or developing countries. Among the drugs that have the greatest dependence on developed countries antineoplastic agents are the most important because of the difficulty of their development, which presents a high degree of technological advance, and the complexity of cancer treatments.4
The entry of these drugs in Brazil faces many barriers such as the pre-clinical and clinical studies of the applicant drug are reviewed in the dossier to confirm that all requirements have been met for the registration of a drug that presents quality, efficacy and safety for the population.
Although these barriers exist, it cannot be ignored that the treatment of cancer is a necessity for thousands of patients in Brazil and represents one of the main causes of death.
Definition and prevalence
Cancer is characterized by a disordered growth of cells that invade tissues and organs, spreading through the body in a process called metastasis. The cells will differentiate according to the site of invasion, and cell multiplication will vary according to the speed of this process.
Cancer is considered a problem of public health in Brazil, aggravated by aging of the population as shown in Table I and Figure 1&2.
Primary Location (Men) |
cases |
% |
Primary Location (Women) |
cases |
% |
Prostate |
65.840 |
29,2% |
Female breast |
66.280 |
29,7% |
Colon and rectum |
20.520 |
9,1% |
Colon and rectum |
20.470 |
9,2% |
Trachea, bronchus, lung |
17.760 |
7,9% |
Cervix |
16.590 |
7,4% |
Stomach |
13.360 |
5,9% |
Trachea, bronchus, lung |
12.440 |
5,6% |
Oral cavity |
11.180 |
5,0% |
Thyroid gland |
11.950 |
5,4% |
Esophagus |
8.690 |
3,9% |
Stomach |
7.870 |
3,5% |
Bladder |
7.590 |
3,4% |
Ovary |
6.650 |
3,0% |
Non-Hodgkin's lymphoma |
6.580 |
2,9% |
Body of the uterus |
6.540 |
2,9% |
Larynx |
6.470 |
2,9% |
Non-Hodgkin's lymphoma |
5.450 |
2,4% |
Leukemia |
5.920 |
2,6% |
Central nervous system |
5.220 |
2,3% |
Table 1 Proportional distribution of the ten types of cancer most estimated for 2020 by sex (adapted from INCA, Brazilian National Center Institute)
*Numbers rounded to multiples of 10.

Figure 1 Worldwide total pharmaceutical R&D spends in 2010-2024.3
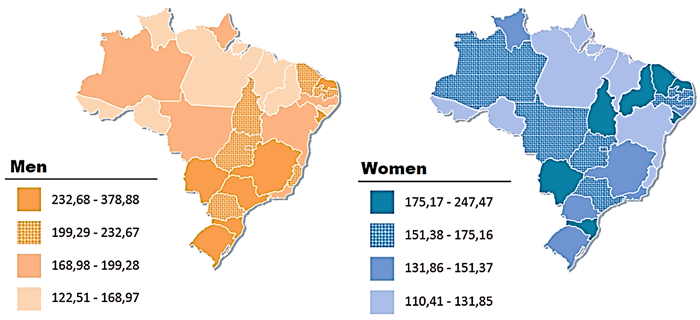
Figure 2Spatial representation of adjusted incidence rates per 100,000 men and women, estimated for the year 2020, according to Federation Unit; all malignant neoplasms, except for non-melanoma skin (adapted from INCA, Brazilian National Center Institute).5
.Regarding the Brazilian situation, the estimate for each year of the 2020-2022 triennium points out that there will be 625 thousand new cases of cancer (450 thousand, excluding cases of non-melanoma skin cancer).5
Summary
In addition, the market for cancer drugs is expanding and still requires a lot of investment in research and innovation, both by private companies and laboratories as well as universities and government. Partnerships between these sectors, such as those currently being carried out in Brazil for the production of biological medicines, should be encouraged so that the advances can be achieved and reach the patients, bringing benefits to their health.
None.
Authors declare there are no conflicts of interests.

©2023 Dezena, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.