Journal of
eISSN: 2473-0831


Research Article Volume 12 Issue 1
1Science Laboratory Technology, Gateway Polytechnic, Sapade, Ogun State, Nigeria
2Nigerian Institute for Trypanosomiasis Research, Vector & Parasitology, Nigeria
Correspondence: Aladenika Yetunde Victoria, Science Laboratory Technology, Gateway Polytechnic, Sapade, Ogun State, Nigeria
Received: December 29, 2022 | Published: January 3, 2023
Citation: Victoria AY, Roland AO. Bioactivities of Launaea taraxacifolia (wild lettuce) on rats induced with osteogenesis inhibitor observed through bone markers. J Anal Pharm Res. 2023;12(1):1-4. DOI: 10.15406/japlr.2023.12.00415
The bioactivities of plants rely solely on the bioactive compounds contained in such plants. Phytochemical extracts of plants such as Launaea taraxacifolia have shown numerous medicinal properties like osteopromotive, antioxidative, anti-inflammatory, anti-cancerous and anti-microbial. The main objectives are to study the osteoroporotic effect of aqueous extract of Launaea taraxacifolia on warfarin-induced rats, where change in body weight, CTX-1, Procollagen type 1 N-terminal Propeptide (P1NP) and procollagen Type 1 C-terminal propeptide (P1CP) level represents the observable parameters during the experiment. Twenty-five (25) adult male Wister albino rats with an average weight of (140g-165g) were obtained for the research. After one weeks of acclimatization, osteosporosis was induced in overnight fasted rats by oral administration at a dosage 0.6ml/ 5mg body weight of Warfarin drug. The effect of induction of warfarin on weight loss showed that reduction was observed in body mass between pre and post induction and weight improved after the treatment. The result also revealed the CTX-1 levels of the induced rats increased significantly P ≤ 0.05 compared to the control group. The Procollagen type 1 N-terminal propeptide (P1NP) level of treated animals decreased when compared to the induced group. The result of Procollagen type 1 C-terminal propeptide also revealed a significant decrease P ≤ 0.05 in its level when compared to the control group. These results suggest that Launaea taraxacifolia may be used during bone recovery after injury and that the leaf should be subjected to more extensive medicinal evaluation.
Keywords: bioactive, osteoporosis, procollagen, bone recovery, medicinal
The therapeutic actions of plants rely solely on the bioactive compounds contained in such plants. Bioactive compounds are synthesized basically from plant primary metabolites such as amino acids, carbohydrates and lipids.1 These phytochemicals produce a specific physiological action, depending on their chemical nature, in human body and can be used for therapeutic purposes. Phytomedicines have been in practice since long time, yet there exists partial knowledge regarding the phytoconstituents responsible for a specific bioactivity and several medicinal plants such as Launaea taraxacifolia, Withania somnifera, Cissus quadrangularis, Tinospora cordifolia etc., and their phytochemical extracts have shown numerous medicinal properties like osteopromotive, antioxidative, anti-inflammatory, anti-cancerous, anti-microbial. This is mainly because of the complexity in the natural product composition and the interactions thereof. Nowadays the research is mainly focusing onto identifying the active phytoconstituent for drug development.2
Bone injuries occur due to various traumatic and disease conditions. Healing of bone injury occurs via a multi-stage intricate process. The field of orthobiologics has provided novel approaches using scaffolds, bioactive molecules, stem cells for the treatment of bone defects. Phyto-bioactives have been widely used in alternative medicine and folklore practices for curing bone ailments. It is believed that different bioactive constituents in plants work synergistically to give the therapeutic efficacy.3 Bioactive molecules in plants extracts act upon different signal transduction pathways aiding in bone healing. Plants extracts seem to be a natural and non-toxic therapeutic alternative in treating bone injuries. Most of the studies on bone healing for these plants have reported oral administration of the extracts and presented them as a safe alternative without any side effects despite giving higher doses.
Launaea taraxacifolia (Wild Lettuce) is a greenish neglected indigenous leafy vegetable among the Yoruba tribe of Southwest, Nigeria. Though it is also locally cultivated in Senegal, Ghana, Dahomey and Sierra Lone.4 It is used as medicine in lactation stimulant, yaws, skeletal structure and food such as salad, cooked in soup and sauces. The leaves of the plant are used to feed cows-in-milk to increase the milk yield and to feed sheep and goat mixed with natron to produce multiple births.5
Bone injuries occur due to various traumatic and disease conditions. Healing of bone injury occurs via a multi- stage intricate process. Body has the potential to rectify most of the bone injuries but some severe traumatic cases with critical size defects may require interventions. The metabolic status of bone can be evaluated using a group of molecules called bone turnover markers (BTMs). BTMs consist of estrogens and structural proteins released from the collagen matrix.6 Although BTMs are divided into 2 groups, formation and resorption markers, some markers allow for evaluation of both formation and resorption. In cases of metabolic bone disease, the balance between bone resorption and formation is impaired; bone resorption and/or bone formation increases or decreases.7 Procollagen type 1 N-terminal propeptide (P1NP) and Procollagen type 1 C-terminal propeptide (P1CP) in the blood have been suggested be one of the reference markers of bone turnover for fracture risk prediction and monitoring of osteoporosis treatment. Telopeptides of type 1 collagens (CTX) is released during collagen degradation and its levels has been reported to be elevated in cases of bone metastasis and in postmenopausal women.8
Phyto-bioactives have been widely used in alternative medicine and folklore practices for curing bone ailments.5 It is believed that different bioactive constituents in plants work synergistically to give the therapeutic efficacy. The aim of this research is to determine the bioactivity of Launaea taraxacifolia on rats induced with osteogenesis inhibitor observed through bone markers.
Materials/Equipment’s/Reagents: Animal cages, weighing balance, dissecting sets, dissecting board, tray, dissecting forceps, hematocrits tubes, Centrifuge and Strip pestle and mortal, board pins, Sterile plain bottle, universal bottles, Carnular syringe pack, Petri dish, latex rubber gloves, paper tapes, cotton wool, Plain A4 paper, Chloroform, Diethylether for anaesthesing, NaCl Buffer, Aqueous solution.
Sample collection: The leaves of Launaea taraxacifolia were harvested in January from Omuooke Ekiti in Ekiti east Local government Area of Ekiti State Nigeria. The plant was identified and authenticated at Federal University of technology Akure (FUTA) herbarium. The plant materials were air-dried and subjected to grinding, then kept in dark air-tight closed containers until they were needed for administration. The powder was dissolved in distilled water and given in doses of 100mg/kg and 200mg/kg body weight respectively by an intragastric tube. This dose was extrapolated from the proposed human therapeutic dose according to Paget and Barnes.6
Experimental animals: Twenty five (25) adult male Wister albino rats with an average weight of (140g-165g) were obtained from the Animal House of Olabisi Onabanjo University Teaching Hospital (OOUTH), on the approval of animal ethnic committee. The rats were divided into groups of five animals each and allowed to acclimatize to experimental condition for a week. They were housed in clean cages and maintained under standard laboratory condition (temperature 252 with dark/light cycle 12/12h). They were fed on rat chow and water was given as desired. The principles of laboratory Animal care as stated by the animal ethic committee was strictly followed throughout the duration of the experiment and they were administered with aqueous leaf extract of Launaea taraxacifolia except the control. After 14 days of administration the various tissues of interest were excised for further analysis.
Extraction/Sample Preparation: Powdered Launaea taraxacifolia hundred and two hundred gram of the ground leaves were dissolved in 250 ml of distilled water respectively. The mixture was filtered with muslin cloth. The filtrate was emptied into a beaker for administration.
Experimental design: All the rats were given warfarin at the dosage of 0.6ml/5mg/kg body weight to induce osteoporosis except the control. Limitations in physical activities, joint swollen and bone loss markers were used to confirm bone degeneration. Treatments were effected by using standard drug Calcium (standard drug), 100mg/kg body weight and 200mg/kg body weight of aqueous leaf extract of Launaea taraxacifolia.
The rats were divided into five groups consisting of 5 rats each which are:
Control – No induction or treatment
Induced group – induced with warfarin
Group 1 – induced and treated with Calcium drug at dosage of100mg/kg body weight.
Group 2 – induced and treated with 100mg/kg body weight of Launaea taraxacifolia tea infusion
Group 3 - induced and treated with 200mg/kg body weight of Launaea taraxacifolia tea infusion
Induction of Experimental rats: All the rats were provided with commercially available rat normal pellet diet and water ad libitum, prior to the experiment. After one weeks of acclimatization, osteosporosis was induced in overnight fasted rats by oral administration at a dosage 0.6ml/ 5mg/kg body weight of Warfarin drug.7 Body weight and osteosporosis were confirmed after induction with warfarin in the present study.
Collection of blood samples for hematological and serum biochemical analysis: Blood samples were collected from the experimental rats via jugular venipuncture and collected in EDTA blood tubes for the determination of hematological parameters and for serum tubes without anticoagulant for the general biochemical and electrolytic parameters, estradiol and BTMs evaluation.
A cell blood count was immediately performed on a Sysmex hematology analyser (Sysmex Europe GmbH, Norderstedt, Germany) device. For serum biochemical analysis, the blood samples were centrifuged at 3000 rpm for 10 minutes and the serum stored in Eppendorf tubes at -20°C for general biochemical parameters, TRAP and estradiol, and at -80°C for analysis of the other BTMs analyses. The general biochemical parameters (Procollagen type 1 N-terminal propeptide (P1NP), Procollagen type 1 C-terminal propeptide (P1CP) and Telopeptides of type 1 collagens (CTX-1)) were measured with commercially available immunoassay kits (ELISA kit, IDS, Boldons, UK).
Statistical analysis: Data are presented as the mean± standard error (SE). Differences between groups and control were analyzed with one-way ANOVA in excel. P ≤ 0.05 was considered statistically significant.
Bone injuries occur due to various traumatic and disease conditions. Weight loss has also been implicated during bone formation/resorption. The table 1 demonstrates the changes in the body weight of experimental groups of male albino rats before induction, after induction and after 14 days of treatment. Weight loss can result from a decrease in body fluid, muscle mass, or fat. A decrease in body fat can be intentionally caused presence of diseases in the body.8
|
Groups |
Weight (g) |
Weight (g) |
Weight (g) |
|
Before induction |
After induction |
After treatment |
|
|
Control |
204.5 |
210.8 |
222.7 |
|
Warfarin Induced |
192.2 |
185.1 |
190.3 |
|
Group 1 |
184 |
159.7 |
172.7 |
|
Group 2 |
174.7 |
130.9 |
163.3 |
|
Group 3 |
195.1 |
179.2 |
180.3 |
Table 1 Effect of leaf extract at different concentration on body weight of albino rats
Induced = untreated rat induced with warfarin.
Group 1 = Calcium administered (100mg/kg body weight).
Group 2= Treated with 100mg/kg body weight tea infusion of Launaea taraxacifolia.
Group 3 = Treated with 200mg/kg body weight tea infusion of Launaea taraxacifolia.
In this study, the injection of warfarin (agent that can cause bone resorption) revealed a significant reduction of the body weight. The weight values improved significantly (p≤ 0.05) upon treatment as seen in the final recorded body weight in animals in treated groups (group 1,2 and 3) including calcium treated, 100mg/kg body weight and 200mg/kg body weight of aqueous leaves extract of Launaea taraxacifolia.
Telopeptides of type 1 collagens (CTX) is released during collagen degradation and its levels has been reported to be elevated in cases of bone metastasis and in postmenopausal women.9 Figure 1 revealed the concentration of type I Collagen Cross-Linked C-Telopeptide (CTX-1) (pg/ml) in induced rats compared to control. CTX-1 is the C-terminal telopeptide (CTX), also known as carboxy-terminal collagen cross links, is used as a biomarker in the serum to measure the rate of bone turnover and it is more specific to bone resorption than any other test currently available.10 In this present study, CTX-1 of induced rats (warfarin induced) increased significantly (p ≤ 0.05) compared to the control rats. The result gotten in these present studies agrees with the research of Marx et al.11 who stated that Lower values represent varying degrees of suppression of normal bone turnover, sometimes also called bone remodeling or bone renewal.
Procollagen type 1 N-terminal propeptide (P1NP) and Procollagen 1 C-terminal propeptide (P1CP) in the blood have been suggested be one of the reference markers of bone turnover for fracture risk prediction and monitoring of osteoporosis treatment. P1NP is formed in the bone and is likely to be elevated in response to bone pathologies including bone infections.12
From Figure 2, the level of Procollagen type 1 N-terminal propeptide (P1NP) (ng/dL) in animals treated with standard drug (calcium), 100mg/kg body weight and 200mg/kg body weight of aqueous extract of Launaea taraxacifolia decreased significantly (p ≤ 0.05) compared to the induced rats (warfarin induced), this is in line with the findings of Nair et al.,12 who observed that the potential value of using Procollagen type 1 N-terminal propeptide (P1NP) P1NP is of particular interest, and state that the increased Procollagen type 1 N-terminal propeptide (P1NP) P1NP in patients with oseosporosis could be due to acute release of the bone formation turnover marker from infected bone.
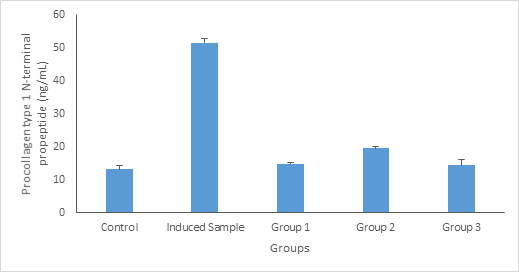
Figure 2 Procollagen type 1 N-terminal propeptide (P1NP) (ng/dL) concentration. Values are given as Mean ±SE of the independent experiment performed in triplicate. Group 1 = Standard drug (Ca); Group 2= Treated with 100mg/kg body weight of Launaea taraxacifolia; Group 3 = Treated with 200mg/kg body weight of Launaea taraxacifolia.
Procollagen type I carboxy-terminal propeptide (PICP), derived from type I procollagen, has been identified as an indicator of type I collagen synthesis in bone matrix formation and skin recovery. From Figure 4.3, the level of Procollagen type 1 C-terminal propeptide in animals treated with standard drug (calcium), 100mg/kg body weight and 200mg/kg body weight of aqueous extract of Launaea taraxacifolia decreased significantly (p ≤ 0.05) compared to the induced rats (warfarin induced), this agrees with previous researches conducted by Burshell et al.,14 who stated that Procollagen type 1 C-terminal propeptide PINP is an extracellular catabolite of type I procollagen and is considered to be a specific biomarker for new bone formation associated with type I collagen synthesis. Figure 3
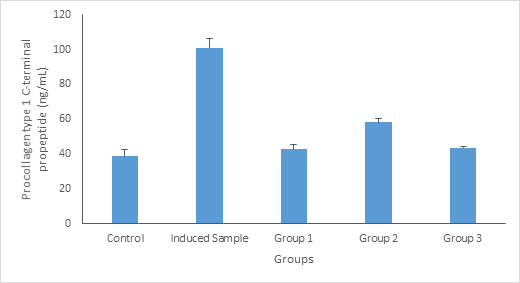
Figure 3 Procollagen type 1 C-terminal propeptide (ng/dL) concentration. Values are given as Mean ±SE of the independent experiment performed in triplicate. Group 1 = Standard drug (Ca); Group 2= Treated with 100mg/kg body weight of Launaea taraxacifolia; Group 3 = Treated with 200mg/kg body weight of Launaea taraxacifolia.
This study demonstrated that the leaves extract of Launaea taraxacifolia helps to improve bone formation by reducing the level of these bone biomarkers in the blood including the Procollagen type 1 N-terminal propeptide (P1NP), Procollagen type 1 C-terminal propeptide (P1CP) and C-Telopeptide CTX levels and in turn the body weight improve drastically. These results suggest that Launaea taraxacifolia may be used during bone recovery after injury.15,16
None.
Authors declare there are no conflicts of interests.

©2023 Victoria, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.