Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 4
Correspondence: Marcellin Guiaro Ndoe, Department of Biomedical Sciences, Faculty of Science, University of Ngaoundere, Ngaoundere, Cameroon
Received: June 28, 2017 | Published: August 6, 2018
Citation: Guiaro MN, Molu JP, Ibrahima D, et al. Beneficial effect of HIV-2 on the progression of HIV-1-associated nephropathy among patients under HAART. J Anal Pharm Res. 2018;7(4):437-444. DOI: 10.15406/japlr.2018.07.00263
This cohort study performed from April 6th, 2017 to May 8th 2018 aimed at optimizing the biomedical follow-up of HIV-associated nephropathy by highlighting the beneficial effect of HIV-2 on the progression of this condition among patients with dual infection (HIV-1 and HIV-2) under Highly Active Antiretroviral Therapy (HAART). Our data show an overall prevalence of 40.4% for HIV-associated nephropathy cases with 30.8% cases for patients with dual infection (HIV-1 and HIV-2) and 69.2% cases for patients only infected with HIV-1 (p=0.001). In this cohort, 257 people were newly diagnosed positive for HIV infection, and displayed a nephrotic syndrome with a focal and segmental glomerulosclerosis more or less associated with a high blood pressure or edema of the lower limbs or a renal insufficiency. These participants registered at the Bertoua day hospital (East Region from Cameroon) for the HAART follow-up. HIV-associated Nephropathy was in most cases, the circumstance of discovery for HIV infection and had a slower progression time among patients with dual infection (HIV-1 and HIV-2) compared to patients only infected with HIV-1. This slow progression time observed among patients with dual infection (HIV-1 and HIV-2) was also positively correlated with a shorter renal function recovery time (7±1.09 months) than the one observed among patients only infected with HIV-1 (11±6.02 months) OR=0.76 [IC 95% (0.18-1.71)] (p=0.05).
Keywords: HIV-associated nephropathy, dual infection HIV-1/HIV-2, HAART, proteinuria
HIV-associated nephropathy is a reality in hospitable environment and is usually diagnosed among patients infected with HIV-1 for several years.1 It is characterized by a focal and segmental glomerulosclerosis (FSGS) accompanied by a massive proteinuria which progress rapidly to an end-stage renal insufficiency.2,3 HIV-associated nephropathy affects black subjects almost exclusively.4,5 The clinical picture is characterized by conflicting clinical situations such as the absence of lower limbs edema despite the severity of the nephrotic syndrome, the absence of high blood pressure and the presence of kidneys with normal size or increased renal ultrasound despite the severity and advanced stage of the renal insufficiency. Classically, HIV-associated nephropathy is a late complication of the infection in a poorly controlled HIV patient with a low CD4 count, a high viral load, and a history of opportunistic infections.1
The incidence of the end-stage renal insufficiency caused by HIV-associated nephropathy does not reflect the many patients with chronic renal insufficiency and/or proteinuria due to HIV-associated nephropathy who have not yet achieved the end-stage renal insufficiency and who therefore do not appear in the national database. The prevalence of HIV-associated nephropathy among HIV-1 seropositive black patients was estimated to be 3.5% in a cohort of HIV-1 patients screened for proteinuria in a primary care setting and up to 12% in a recent autopsy series.6,7 According to the Centers for Disease Control, there are about 140.000 Afro-Americans currently living with AIDS.7 These data suggest that there are between 4.900 and 17.000 black patients in the United States infected with HIV. The prevalence of HIV-1 infection and AIDS is increasing in the United States, particularly among black patients, thus continually increasing the group of most at risk patients to develop HIV-associated nephropathy.8 Among 40 million people living with HIV/AIDS in the world, 28.5 million live in sub-Saharan Africa.9 The incidence of HIV-associated nephropathy in Africa, however, is unknown. Assuming that the prevalence of HIV-associated nephropathy in black patients in this region is similar to the one of black HIV-1 infected patients in the United States, it could be predicted that there are between 1 and 3.4 million cases of HIV-associated nephropathy in sub-Saharan Africa. It is likely that the lack of HIV-associated nephropathy publications in Africa is linked to multiple factors, including a lack of surveillance and reporting for kidney disease. HIV-associated nephropathy is usually a late manifestation of HIV infection.10 As a result, it is likely that many Africans with AIDS die of opportunistic infections in early AIDS, before HIV-associated nephropathy becomes clinically evident. It is foreseeable that as medical care for HIV-infected Africans improves and patients live longer with AIDS, HIV-associated nephropathy will become an increasingly important cause of morbidity and mortality in Africa.
HIV-1 and HIV-2 are the two retroviruses which cause Acquired Immunodeficiency Syndrome (AIDS). However, compared to HIV-1 infection, HIV-2 infection is characterized by lower transmission rates, a longer asymptomatic stage, a slower decline in CD4 count+T cell numbers and a lower mortality rate. Progressive immune dysfunction and AIDS are developing in most people who are infected only with HIV-1, compared to only 20-30% of those infected with HIV-2.10,11 In West Africa, an area with a high prevalence of HIV-2, the prevalence of HIV-1 and HIV-2 dual infection is 0 to 3.2%.12,13 In 1995, a potential protective effect of HIV-2 against subsequent HIV-1 infection among sex workers in Senegal was reported.14 This protective effect could not be verified in other cohorts.15,16
Several studies have reported that HIV-2 infection can alter HIV-1 infectivity and replication in vitro.17,18 This study enters this viewpoint in the context of HIV-associated nephropathy by highlighting the beneficial effect of HIV-2 on its progression among patients with dual infection (HIV-1 and HIV-2).
Study design, sampling and target population
We carried out a cohort study with prospective data collection from April 6th 2017 to May 8th 2018 on 257 newly diagnosed HIV-positive patients with HIV-associated nephropathy at the Bertoua Regional Hospital, specifically in its medical consultation services, hemodialysis center and day hospital.
Ethical consideration
The administrative authorization of the research and the ethical clearance obtained respectively from the Ministry of Public Health and the National Committee of Ethics of Research for Human Health from Cameroon, served as a springboard to gain access easy in our study place. In addition, informed and signed consent was obtained from our participants prior to the start of the data collection of our study.
Criteria for inclusion and exclusion
We included in our study, all newly diagnosed HIV-positive patients, and registered for the HAART follow-up at the Bertoua day hospital and presenting a nephrotic syndrome with a focal and segmental glomerulosclerosis more or less associated with a high blood pressure or lower limbs edema or a renal insufficiency. We excluded from our study, pregnant women, diabetic patients, sickle cell patients, viral hepatitis cases, urinary schistosomiasis (history of hematuria), patients on long-term non-steroidal anti-inflammatory, patients with a history of kidney disease, cancer cases, patients with HIV-negative status as well as participants who refused to sign informed consent to participate in the study.
HIV serology and biological diagnosis of HIV-associated nephropathy
The HIV serology was performed by a rapid test (Determine HIV-1 and 2) and the diagnosis of HIV-associated nephropathy, in the absence of renal biopsy, was evoked on the basis of proteinuria greater than three grams per day with or without hematuria (red blood cells >10/mm3 deformed or cylindrical), with or without renal insufficiency, in a HIV-infected patient. We performed for all patients a proteinuria on the urine of 24 hours. In case of proteinuria greater than three grams per day, a cytobacteriological urinary examination has been carried out systematically, as well as the search for another infectious focus point.
Creatinine clearance and affected renal function
Creatinine clearance was estimated using the Cockcroft and Gault formula:
For men: Ccr (ml/min) = (140-age) x weight (kg) x 1.23/plasma creatinine (micromole/l)
For women: Ccr (ml/min) = (140-age) x weight (kg) x 1.05/plasma creatinine (micromole/l)
The affected renal function has been classified according to the following Table 1:19
Stage |
GFR (ml/min) |
Definition |
1 |
>90 |
Kidney injury with normal or increased GFR |
2 |
60-89 |
Kidney injury with slightly decreased GFR |
3 |
30-59 |
GFR moderately decreased |
4 |
15-29 |
GFR severely decreased |
5 |
<15 |
End stage renal insufficiency |
Table 1 Classification of the affected renal function according to the glomerular filtration rate (GFR):21
GFR, glomerular filtration rate
Therapeutic protocol
The participants in our study received a corticosteroid with a conversion enzyme inhibitor associated with HAART without protease inhibitor: d4T-3TC+NVP or EFV and AZT-3TC+NVP or EFV for the group of patients only infected with HIV-1 and AZT+3TC+ABC for the group of patients with dual infection (HIV-1 and HIV-2).
Data collection
The participants in our study were recruited randomly in medical consultation services and hemodialysis center from the Regional hospital of Bertoua. For each participant, we collected sociodemographic data (age and sex), biological data (viral load, type of HIV, CD4 count, uremia, serum creatinine, creatinine clearance, glomerular filtration rate, proteinuria, hematuria), clinical data (hypertension, renal insufficiency, edema, clinical stage of HIV infection) and therapeutic data (therapeutic protocol). The follow-up for HIV-associated nephropathy was done quarterly through the control of biological parameters cited above of participants under HAART with corticosteroid associated with a conversion enzyme inhibitor.
Statistical analysis
The data collected were analyzed with Excel and R version 2.13 software. The Chi-square, Fisher and ANOVA tests were used to compare the percentages and means of results obtained with a threshold value of 5 (Figure 1).
We found 636 cases of nephrotic syndromes during our study period, of which 257 cases of HIV-associated nephropathy were registered and included in our study, that is a prevalence of 40.4% with 79(30.8%) cases for patients with dual infection (HIV-1 and HIV-2) and 178(69.2%) cases for patients only infected with HIV-1 (p= 0.001).
Sociodemographic data
The overall sex-ratio was 0.29 in favor of women who were the most represented with 73.5% and 78.7% respectively for the group of patients with dual infection (HIV-1 and HIV-2) and the group of patients only infected with HIV-1. The overall average age was 46.74±6.95 years ranged from 21 to 72 years. The cases of HIV-associated nephropathy appeared to increase with age. The age group of 50 years and above was the most represented with 52.1% and 60.3% (p=0.01) respectively for the group of patients with dual infection (HIV-1 and HIV-2) and the group of patients only infected with HIV-1 (Table 2).
|
HIV-associated nephropathy cases |
|
||||
Sociodemographic parameters |
Patients with dual infection (HIV-1+2) |
Patients only infected with HIV-1 |
|
|||
n |
% |
n |
% |
p |
||
Age groups |
[20-30] |
8 |
10.10% |
19 |
10.60% |
0.001 |
(In years) |
[30-40] |
12 |
15.10% |
23 |
12.90% |
0.03 |
[40-50] |
18 |
22.70% |
29 |
16.20% |
0.01 |
|
[³ 50] |
41 |
52.10% |
107 |
60.30% |
0.01 |
|
Total |
79 |
100% |
178 |
100% |
- |
|
Sex |
Male |
21 |
26.50% |
38 |
21.30% |
0.04 |
Female |
58 |
73.50% |
140 |
78.70% |
0.001 |
|
Total |
79 |
100% |
178 |
100% |
- |
|
Table 2 Distribution of HIV-associated nephropathy cases according to sociodemographic data
Damage’level of the renal function
All patients with dual infection (HIV-1 and HIV-2) had a nephropathy with a beginner renal insufficiency compared to patients only infected with HIV-1 who presented an advanced renal insufficiency in 96.6% of cases (Table 3) (Figure 2).
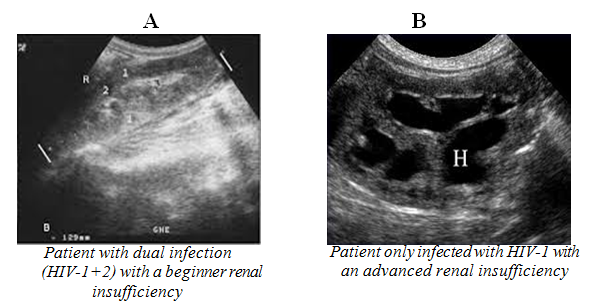
Figure 2 Renal ultrasound of a beginner and an advanced renal insufficiency respectively for a patient with dual infection (HIV-1+2)”A” and a patient only infected with HIV-1 “B”.
|
HIV-associated nephropathy cases |
|
|||
Patients with dual infections (HIV-1+2) |
Patients only infected |
|
|||
Damage’level of the renal function |
n |
% |
n |
% |
p |
Beginner RI |
79 |
100% |
6 |
3.37% |
0.001 |
Advanced RI |
0 |
0 |
172 |
96.63% |
0.03 |
Terminale RI |
0 |
0 |
0 |
0 |
- |
Total |
79 |
100% |
178 |
100% |
- |
Table 3 Distribution of HIV-associated nephropathy cases according to the damage’level of the renal function
RI, renal insufficiency
Therapeutic data
Therapeutic protocol: All patients with dual infection (HIV-1 and HIV-2) received AZT-3TC-ABC, that is 79 patients for a rate of 31% (p= 0.0001) (Table 4).
Therapeutic Protocol |
Numbers |
Percentages |
d4T-3TC-NVP |
58 |
22.5% |
d4T-3TC-EFV |
35 |
13.6% |
AZT-3TC-NVP |
46 |
17.8% |
AZT-3TC-EFV |
39 |
15.1% |
Total |
257 |
100% |
Table 4 Distribution of participants in our study according to the therapeutic protocol
D4T, stavudine; AZT, zidovudine; 3TC, lamivudine; ABC, abacavir
Biological data
Case of patients only infected with HIV-1 : The average recovery time for the renal function of patients only infected with HIV-1 was 11±6.02 months, with a creatinine clearance and a CD4 count presenting respectively average increases of 62±6.14ml/min and 316±2.7cells/mm3 of blood (p=0.02) OR=0.60 [95% CI (0.17-1.98)]. They were also correlated with a significant decrease of the viral load, uremia, serum creatinine, proteinuria and hematuria with respectively average decreases of 123363±14549 copies/ml, 40.75±3.9/l, 50.4±18.9μmol/l, 5.47±4.24g/24h and 7.88±2.2cells/mm3 (Table 5).
Viral load |
Uremia (µmol/l) |
Serum Creatinine (µmol/l) |
Creatinine Clearance (ml/min) |
CD4 (cells/mm3) |
Proteinuria (g/24h) |
Hematuria (cells/mm3) |
OR (CI - 95%) |
p |
|
Months |
Values (Copies/ml) |
|
|||||||
Before HAART |
126845±14787 |
193.56±14.2 |
198.7±10.2 |
23.4±18.2 |
165±7.4 |
9.36±5.3 |
18.76±4.6 |
0.99 (0.18-1.19) |
0.03 |
3 Months |
88118±5067 |
188.1±12.09 |
192.18±14.6 |
32.6±6.1 |
353±5.6 |
6.38±2.9 |
15.29±3.01 |
0.8 (0.12-1.26) |
0.01 |
6 Months |
11141±394 |
163.89±14.6 |
173.26±29.8 |
59.2±9.3 |
365±8.5 |
5.96±3.1 |
13.41±9.6 |
0,79 (0.36- 1.86) |
0.04 |
9 Months |
3482±238 |
152.81±26.3 |
148.3±29.1 |
85.4±12.06 |
481±10.1 |
3.89±1.06 |
10.88±6.8 |
0.6 (0.17-1.98) |
0.02 |
12 Months |
<40 |
123.17±18.1 |
121.2±6.8 |
93.7±21.4 |
586±9.02 |
3.21±1.8 |
10.69±4.01 |
0.72 (0.29-1.77) |
0.001 |
r |
0.12 |
0.003 |
0.62 |
0.0004 |
0.42 |
0.13 |
- |
- |
|
Table 5 Distribution of biological parameters of patients only infected with HIV-1 according their evolution during the HAART follow-up
OR, odds ratio; CI, confidence interval; r, correlation coefficient
Case of patients with dual infection (HIV-1 and HIV-2)
The mean recovery time for the renal function of patients with dual infection (HIV-1 and HIV-2) was 7±1.09 months, with creatinine clearance and a CD4 count presenting with average increases of 18.7 ± 5.4ml/min and 277±11.6cells/mm3 of blood (p=0.05) OR=0.76 [95% CI (0.18-1.71)]. They were also correlated with a significant decrease of the viral load, uremia, serum creatinine, proteinuria, and hematuria with average decreases of 1528953±20693 copies/ml, 26.5±10.2μmol/1, 26.5±0.4μmol/l, 3.4±0.2g/24h and 9.8±3.4 cells/mm3 (Table 6).
Viral load |
Uremia (µmol/l) |
Serum Creatinine (µmol/l) |
Creatinine Clearance (ml/min) |
CD4 (cells/mm3) |
Proteinuria (g/24h) |
Hematuria (cells/mm3) |
OR (CI - 95%) |
p |
|
Months |
Values (Copies/ml) |
|
|
|
|
|
|
|
|
Before HAART |
1532452±21391 |
159.2±29.6 |
162.8±9.4 |
23.4±18.2 |
165±7.4 |
9.36±5.3 |
24.6±2.7 |
0.74 (0.16-1.81) |
0.001 |
|
|
||||||||
3 Months |
10118±8493 |
146.8±36.1 |
152.9±11.2 |
32.6±6.1 |
353±5.6 |
6.38±2.9 |
19.6±8.4 |
0.9 (0.47-1.08) |
0.18 |
6 Months |
3499±698 |
132.7±19.4 |
136.3±9.8 |
59.2±9.3 |
365±8.5 |
5.96±3.1 |
14.8±6.1 |
0.84 (0.57-1.62) |
0.02 |
9 Months |
<40 |
121.9±8.3 |
129.3±5.3 |
85.4±12.06 |
481±10.1 |
3.89±1.06 |
12.1±3.6 |
0.76 (0.18-1.71) |
0.05 |
12 Months |
<40 |
115.5±6.3 |
118.7±4.8 |
93.7±21.4 |
586±9.02 |
3.21±1.8 |
10.2±9.1 |
0.56 (0.39-1.91) |
0.01 |
r |
0.06 |
0.01 |
0.62 |
0.0004 |
0.42 |
0.02 |
- |
- |
|
Table 6 Distribution of biological parameters of patients with dual infection (HIV-1 and HIV-2) according their evolution during the HAART follow-up
OR, odds ratio; CI, confidence interval; r, correlation coefficient
Glomerular filtration rate (GFR) of drugs
The glomerular filtration rate increased after 3 months regardless of the HAART administered (Table 7).
Treatment |
d4T-3TC-NVP |
d4T-3TC-EFV |
AZT-3TC-NVP |
AZT-3TC-EFV |
AZT-3TC-ABC |
GFR before HAART ml/min |
62.2 ±6.1 |
67.9±26.3 |
60.1±52.3 |
66.4±9.08 |
65.8±11.2 |
GFR at 3 months |
69.7±9.03 |
72.05±8.02 |
64.1±18.6 |
72.3±5.8 |
69.5±9.8 |
GFR at 6 months |
72.4±5.8 |
78.5±21.2 |
69.8±42.8 |
76.1±9.3 |
71.05±8.1 |
GFR at 9 months |
83.2±19.6 |
81.01±10.3 |
77.6±35.1 |
81.6±13.2 |
76.8±4.9 |
GFR at 12 months |
96.8±8.9 |
98.9±9.3 |
98.09±8.9 |
93.9±7.6 |
99.01±9.4 |
p |
0.01 |
0.003 |
0.05 |
0.02 |
0.001 |
Table 7 Distribution of Participants according to the glomerular filtration rate of drugs
GFR, glomerular filtration rate
Evolution of HIV-associated nephropathy under HAART
All participants in our study showed a statistically significant improvement in their health status after 12 months of HAART (p=0.05) (Table 8).
|
HIV-associated nephropathy cases |
|||
Patients with dual infections |
Patients only infected |
|||
Damage’level of the renal function |
n |
% |
n |
% |
Nearly normal kidney |
75 |
96.16% |
0 |
0 |
Nephropthy without RI |
3 |
3.84% |
176 |
98.80% |
Beginner RI |
0 |
0 |
2 |
1.20% |
Moderate RI |
0 |
0 |
0 |
0 |
Severe RI |
0 |
0 |
0 |
0 |
End stage RI |
0 |
0 |
0 |
0 |
Deaths |
0 |
0 |
0 |
0 |
Total |
78 |
100% |
178 |
100% |
Table 8 Distribution of participants according to the evolution of HIV-associated nephropathy under HAART
RI, renal insufficiency
Our study reported 257 cases of HIV-associated nephropathy in 636 cases of nephrotic syndrome registered during our study period. That is an overall prevalence of 40.4%. Among these 257 cases HIV-associated nephropathy, 79(30.8%) cases had a dual infection (HIV-1 and HIV-2) and 178(69.2%) cases were only infected with HIV-1. The overall prevalence of 40.4% cases of HIV-associated nephropathy, reported by our study, is well above the range of prevalence found in studies made by Rao, Pardo, Gardenswartz, and Langs which were ranged from 10% to 20,3,20–22 and reflects the existence of a hidden epidemic of people living with HIV (PLHIV) who are unaware of their HIV status. These people can transmit HIV because, being unaware of their infection, they are less able to adopt preventive behaviors aimed at not transmitting the virus, and above all they do not benefit from HAART which reduces the risk of transmission.23,24
The average age was 46.74±6.95 years ranged from 21 to 72 years. The cases of HIV-associated nephropathy appeared to increase with age. The age group of 50 years and above was the most represented with 52.1% and 60.3% (p=0.01) respectively for the group of patients with dual infection (HIV-1 and HIV-2) and the one of patients only infected with HIV-1 (Table 2). This confirms the late diagnosis of HIV infection and the non-compliance with the rules of prevention emanating from the awareness of HIV infection by the population and also the lack of follow-up of this population by the Ministry of Public Health because nephropathy was in the most cases, the circumstance of discovery for HIV infection.
The overall sex ratio was 0.63 in favor of women, who were the most represented with 73.5% and 78.7% (p= 0.001) respectively for patients with dual infection (HIV-1 and HIV-2) and the one of patients only infected with HIV-1 (Table 2). This shows the susceptibility of the woman to HIV infection and indirectly to HIV-associated nephropathy. This susceptibility is probably related to anatomical and physiological predispositions. This observation follows the trend of the feminization of the infection described by EDS III 2010 (6.8% versus 4.1%);22 by the 2011 DHS-MICS (5.6% versus 2.9%);25 This is also true for the Mbopi-Keou study, where there was a change in HIV prevalence among the female population, from 6.7% in 2005 to 9.73% in 2008.26
79(30.8%) patients among the 257 cases of HIV-associated nephropathy had a dual infection (HIV-1 and HIV-2) compared with 178(69.2%) cases for patients only infected with HIV-1. Patients with dual infection (HIV-1 and HIV-2) had a nephropathy with a beginner renal insufficiency compared to those only infected with HIV-1 who presented an advanced renal insufficiency in 96.6% of cases (Table 3). This difference observed among patients with dual infection (HIV-1 and HIV-2) could be explained by the low replicative capacity of HIV-1 in case of coinfection with HIV-2.27 Thus, HIV-1 replication more virulent and faster at the renal level and in the absence of coinfection with HIV-2, leads to cellular changes (apoptosis and phenotypic changes in epithelial cells) and the synthesis of a high rate of inflammatory mediators (including TGFβ and TNFα). These two mechanisms lead to the rapid progression of lesions observed towards the end stage of the renal insufficiency.28,29 In vitro studies have shown that HIV-2 infection generates higher levels of natural beta-chemokine ligands than CCR5 HIV co-receptor mononuclear peripheral blood cells and that this may inhibit HIV-1 infection and its replication at the renal level.20,28,29
The average recovery time for the renal function and also the disappearance of renal lesions in the group of patients with dual infection (HIV-1 and HIV-2) was 7±1.09 months after initiation of HAART (Table 6) (Table 7). It was significantly shorter than the one observed in the group of patients only infected with HIV-1 which was 11±6.02 months (p = 0.05) OR=0.76 [95% CI (0.18-1.71)] (Table 5) (Table 7). This faster recovery time of the renal function and disappearance of renal lesions during the HAART in the group of patients with dual infection (HIV-1 and HIV-2) would reflect the beneficial effect of HIV-2 on HIV-1 infection and its replication at the renal level causing the lesions observed in cases of nephropathy.30–32 It also highlights the effectiveness of HAART on HIV-associated nephropathy. This finding is similar to those reported in studies conducted by Wali and Kirchner on the beneficial effect of antiretroviral therapy on HIV-associated nephropathy.31,32 The evolution of HIV-associated nephropathy under HAART was statistically significant in both patients groups (p=0.04). 96.16% of patients with dual infection (HIV-1 and HIV-2) returned to a nearly normal renal physiology after 12 months of HAART and 98.8% of patients only infected with HIV-1 progressed from a renal insufficiency advanced to a nephropathy without renal insufficiency (Table 8).
Our study despite its limits namely, the low number of study participants (Patients with dual infections HIV-1+ HIV-2), short follow-up times and lack of data on the timing of infection, has reported a prevalence of 40.4% of HIV-associated nephropathy cases with 30.8% for cases with patients presenting a dual infection (HIV-1+HIV-2) compared to 69.2% for cases of patients only infected with HIV-1. We noted during this study that the progression of HIV-associated nephropathy cases in the group of patients with dual infection was slower than the one observed in the group of patients only infected with HIV-1, possibly because of the low replicative capacity of HIV-1 in case of coinfection with HIV-2. This slow progression of HIV-associated nephropathy cases in the group of patients with dual infection (HIV-1+ HIV-2), was strongly correlated with a faster recovery time for the renal function than the one observed in the group of patients only infected with HIV-1, OR = 0,76 [95% CI (0.18-1.71)]. The prevalence of 40.4% of HIV-associated nephropathy cases reported in our study is well above the 4.3% prevalence of HIV infection in Cameroon33 and reflects the existence of a hidden epidemic of people living with HIV (PLHIV) who are unaware of their HIV status because HIV-associated nephropathy was in the most cases the circumstance of discovery for HIV infection of participants in our study. As a result, the Ministry of Public Health should focus on the follow-up of the population after the actions undertaken to raise awareness about the diagnosis and prevention of HIV infection in Cameroon in order to reduce or limit cases of HIV infection and indirectly HIV-associated nephropathy cases.
All authors contributed to the designing, preparation, editing and final review of the manuscript.
Authors thank the collaborators of their respective institutions for the comments on the manuscript and the study participants.
The author declares that there is no conflict of interest.

©2018 Guiaro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.