Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 3
Correspondence: Abdalla Ahmed Elbashir, Department of Chemistry, Faculty of science, University of Khartoum, Sudan
Received: April 27, 2016 | Published: May 4, 2016
Citation: Rahman STA, Elbashir AA, El-Mukhtar M, Ibrahim MM (2016) Application of Spectrophotometric Methods for the Determination of Thiamine (VB1) in Pharmaceutical Formulations Using 7-Chloro-4-Nitrobenzoxadiazole (NBD-Cl). J Anal Pharm Res 2(3): 00018. DOI: 10.15406/japlr.2016.02.00018
Simple, swift and sensitive method for Spectrophotometric determination of thiamine (VB1) in pharmaceutical tablets has been described. The proposed method is based on the reaction of thiamine (VB1) and 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl) at alkaline medium (pH 10.5) to develop a deep brown adduct that bears maximum absorption at 434 nm. Beer’s law is obeyed in the concentration range 5-35 µg/ml of thiamine at the selected wavelength. Under optimized reaction conditions, the linear regression coefficients were a=0.033, b=-0.009, and r2=0.999 calculated for the general equation of the calibration curve (y = ax + b).
The limit of detection (LOD) and limit of quantification (LOQ) were found to be 0.667µg/ml and 2.020µg/ml respectively. The method has been successfully applied to the determination of thiamine (VB1) in pharmaceutical formulations, and can be used as an alternative of the existing sophisticated method used in drug laboratories and factories.
Keywords:thiamine (VB1), spectrophotometric, pharmaceutical formulation, 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl)
LOD, limit of detection; LOQ, limit of quantification; VB1, Thiamine; FIA, flow injection analysis; NBD-Cl, 7-Chloro-4-Nitrobenzoxadiazole; ICH, international conference on harmonization; HPLC, high-performance liquid chromatography; RE, relative error
Since Thiamine - the first discovered vitamin among B complex vitamins - is considered as an essential nutrient,1 and used for treatment of many disorders and diseases;2 and because it is included as a component of different multi-vitamins, a variety of analytical methods have been proposed for its determination in pharmaceutical formulations. Those methods include flow injection analysis (FIA),3-7 spectrophotometric8 chromatographic methods,9,10 fluorometry,11 high-performance liquid chromatography (HPLC),12,13 chemilumenescence,2 and electrochemical techniques.14,15
Most of those methods, though sensitive and reliable, are associated with several disadvantages. FIA methods require a pre-oxidation step of thiamine and extraction with chloroform.7 Other techniques such as HPLC and electrochemical techniques require highly sophisticated instrumentation besides tedious and time-consuming experimental steps. This sheds light to the need of alternative simple and sensitive methods for thiamine analysis.
Spectrophotometry is considered the most convenient analytical technique, because of its inherent simplicity, low cost, and wide availability in most quality control laboratories.16 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl) is a well known fluorogenic and color-forming reagent that is widely used in the analysis of a variety of amino group containing drugs.17-20 Its use in pharmaceutical analysis was reviewed by Elbashir et al.17 and proved to be a convenient low-cost derivatizing reagent.21,22
Thus far, the reaction between Thiamine and NBD-Cl has not been reported yet. In a previous paper, we reported a sensitive and simple Spectrophotometric procedure for determination of Thiamine using NQS as color-developing reagent.8 In this context we report the determination of Thiamine using NBD-Cl as a reagent, and we establish the optimum reaction conditions necessary for reliable measurements in dosage forms.
Apparatus
Absorbance measurements were carried out using Shimadzu UV-Visible Spectrophotometer model 1800 with quartz cells of 1cm optical path length, pH measurements were performed in pH meter model HI 255 (Hanna Instruments, Mumbai, India). Masses were taken using a digital Analytical Balance.
Material and reagent
Thiamine hydrochloride was obtained from Sigma Aldrich Ltd, Khartoum, Sudan, and used as received; its purity was 99.15%. A solution of 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl) 0.2% (w/v) was prepared by dissolving 0.2g in distilled water, transferred into a 100mL volumetric flask and diluted to the mark with distilled water then mixed well. The solution was freshly prepared and protected from light during use. A series of buffer solutions of pH range from 7-12 were prepared according to the literature methods.23 An optimum buffer solution of pH 10.5 was prepared by mixing 100ml of 0.025M aqueous solution of Borax with 36.5ml of 1M solution of sodium hydroxide and adjusted to pH 10.5 with 1M Sodium hydroxide. All other chemicals were of analytical grade.
Stock standard solution of thiamine (1000μg/mL)
An accurately 250mg of thiamine hydrochloride standard was dissolved in distilled water, transferred into 250 ml volumetric flask, diluted to the mark with same solvent and mixed well. This stock solution was further diluted to 200μg/mL to obtain working solutions in the range of 5-35μg/mL.
Tablets sample solution
Five capsules (Thiamine 100mg/capsule) were weighted and finely-grinded. A portion of the powder equivalent to 25mg of the drug was weighted and dissolved in distilled water, filtered and then transferred into 250 volumetric flask, completed to the mark with distilled water to give a solution of 100 µg/mL. The solution was then subjected to chemical analysis according to the following procedure.
Procedure of analysis
Aliquots of 200μg/mL of Thiamine was transferred into 10ml volumetric flask to give final concentrations of 5-35μg/mL, 1.5mL of 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl) 0.2% (w/v) was added and followed by 1.5mL pH 10.5 buffer solution. The reaction was completed to volume with distilled water, and the resulting solution was measured at 434nm against reagent blank treated similarly.
Stoichiometry of the chemical reaction
The method of continuous variation (Job’s method): The Job’s method of continuous variation was employed to determine the stoichiometric ratio of the reaction between thiamine and NBD-Cl.23 Equimolar (7.5×10-4M) aqueous solution of Thiamine hyrochloride and NBD-Cl were prepared. A series of 10 mL portions of Thiamine standard and NBD-Cl were made up comprising different complementary proportions (1:9,…9:1, inclusive) in 10 mL volumetric flask containing 1.5 ml of buffer solution (pH=10.5). The solution was further manipulated as described under the general recommended procedures.24
Absorption spectra
The absorption spectrum of thiamine was recorded against water (Figure 1), it was found that thiamine exhibits a maximum absorption peak (λ max) at 235nm. Because of highly blue-shifted λ max of thiamine, its determination in the dosage form based on the direct measurement of its absorption for ultraviolet is susceptible to potential interferences from the common excipients. Therefore, derivatization of thiamine to attain visible-range absorbing species was undoubtedly necessary. Thus, derivatization of thiamine with NBD-Cl was performed, and the absorption spectrum of the product was recorded against reagent blank (Figure 1). It was found that the product is brown colored exhibiting λmax at 434nm, and the λmax of NBD-Cl was 342nm. The λmax of thiamine-NBD-Cl derivative was red-shifted, eliminating any potential interference. The wavelength 434 nm therefore was fixed as optimum.
Optimization of the reaction conditions
The optimum conditions for the developed method were established by varying the parameters one at a time while keeping the other parameters constant and following the effect exerted on the absorbance of the colored product. In order to establish experimental conditions, the effect of various parameters such as pH, time, buffer volume and concentration of NBD-Cl were investigated.
Effect of pH
The effect of pH on the reaction between thiamine and NBD-Cl was tested by varying the pH form 7.0 to 12.0. As shown in Figure 2, the absorbance of the product is low at pH 7.0, indicating that thiamine cannot react with (NBD-Cl) in neutral media. This was possibly due to the existence of the amino group of thiamine in the form of hydrochloride salt, which hampers nucleophilic substitution capability. As the pH increased from 7 to 12, the readings increased dramatically, releasing the amino group of thiamine and facilitates the nucleophilic substitution. The maximum absorption was attained at pH value of 10.5. At pH values more than 10.5, a decrease in the absorption occurred. This was attributed probably to the increase in the amount of hydroxide ion that increases the rate of the backward reaction of thiamine with NBD-Cl.
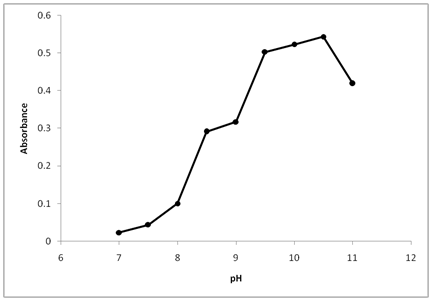
Figure 2 Effect of pH on the reaction of Thiamine with NBD-Cl. Thiamine (20µg/ml): 1 ml, NBD-Cl conc. 0.2% (w/v), reaction time 20 min.
Effect of reaction time
The absorbance of the reaction product was monitored at different times (Figure 3). Keeping other conditions intact, the absorbance of the reaction product was followed after standing for different time spans at 25°C. The results show that thiamine reacts with NBD-Cl at 25°C and the absorbance begins to increase gradually and reach a maximum after 25min. For longer reaction times, a slight drop in the absorbance was observed. Accordingly 25min was set as the convenient reaction time for determination.
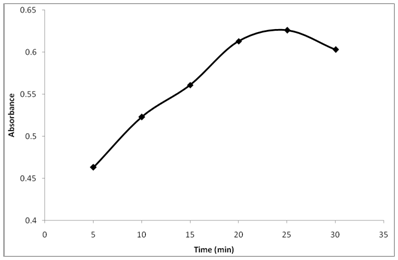
Figure 3 Effect of reaction time on the reaction of Thiamine with NBD-Cl. Thiamine (20µg/ml): 1 ml, Buffer (pH 10.5): 1.5 ml, NBD-Cl conc. 0.2% (w/v).
Effect of amount of the buffer
Keeping pH at 10.5, the effect of amount of buffer solution on the absorbance of reaction product was also studied (Figure 4). The figure reveals that the absorbance of the reaction product enhances rapidly with the rise of amount of buffer solution, and becomes maximal when the amount of buffer solution reaches 1.5 mL. Therefore, the amount of 1.5mL buffer solution was selected to ensure the highest absorbance.
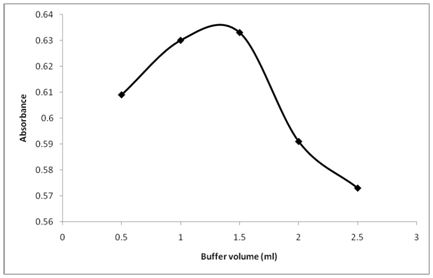
Figure 4 Effect of Buffer amount (ml) on the reaction of Thiamine with NBD-Cl. Thiamine (20µg/ml): 1 ml, Buffer pH: 10.5, NBD-Cl conc. 0.2% (w/v), reaction time 25 min.
Effect of NBD-Cl concentration
The study of the effect of NBD-Cl concentrations showed that the reaction was dependent on the reagent concentration. The highest absorption intensity was attained at NBD-Cl concentration of 0.2% (w/v), and higher concentration of NBD-Cl leads to a decrease in the absorbance (Figure 5). From the above parameters-adjusting experiments, the optimized conditions used for the assay were: pH 10.5, NBD-Cl concentration 0.2% (w/v), volume of the buffer 1.5mL, reaction time 25min and temperature 25ºC.
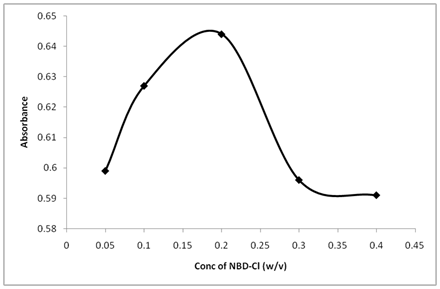
Figure 5 Effect of NBD-Cl concentration on its reaction with Thiamine. Thiamine (20µg/ml): 1 ml, Buffer (pH 10.5): 1.5 ml, reaction time 25 min.
Validation of the method
The method was validated for the following parameters: specificity, linearity, precision, accuracy, limit of detection (LOD), limit of quantitation (LOQ), and robustness according to the International Conference on Harmonization (ICH) guidelines.25
Linearity, limit of detection (LOD) limit of quantification (LOQ)
The linearity was evaluated by linear regression analysis determined by constructing seven concentrations of thiamine, in the range of 05–35μg/mL, which was calculated by the least square regression method to calculate the calibration equation and the correlation coefficient. The calibration curves were constructed by plotting concentration versus absorbance, using linear regression analysis. The regression equation for the results was A=0.033x - 0.009 (r2=0.999), where A is the absorbance at 434 nm, x is the concentration of thiamine in μg/mL in the range of 05-35 μg/mL, and r is correlation coefficient (Table 1). It was found that the linear concentration range is comparable with our previous method using NQS.8 The limit of detection (LOD) and limit of quantification (LOQ) were determined according to the following formula LOD=3.3×SDa/b, and LOQ=10×SDa/b, SDa is the standard deviation of the intercept; b is the slope under the ICH guidelines.25 The LOD and LOQ were found to be 0.667 and 2.020μg/mL, respectively (Table 1).
|
Parameter |
Value |
|
Measurement wavelength (nm) |
434 |
|
Linear range (μg/mL) |
May-35 |
|
Regression equation |
y = 0.033x - 0.009 |
|
Intercept |
-0.00986 |
|
Standard deviation of intercept |
0.00667 |
|
Slope |
0.033 |
|
Standard deviation of slope |
0.000298 |
|
Correlation coefficient (r2) |
0.9995 |
|
Limit of detection, LOD (μg/mL) |
0.667 |
|
Limit of quant., LOQ (μg/mL) |
2.0207 |
Table 1 Parameters for the performance of the proposed method
Accuracy
The accuracy of the proposed method was carried out by applying 3 different concentrations 10, 20, and 30μg/mL of thiamine drug within linear range calculated as the percentage of the drug recovered from the samples (Table 2).
|
Sample No |
Sample Content (µg/mL) |
Thiamine Standard Amount (µg/mL) |
Amount Found (Total) (µg/ml) |
Recovery ±SD* |
|
1 |
5 |
10 |
14.5 |
96.29±0.015 |
|
2 |
5 |
20 |
24.72 |
98.90±0.018 |
|
3 |
5 |
30 |
35.5 |
101.47±0.148 |
Table 2 Recovery studies for the determination of thiamine by the proposed method
*Values are mean of three determinations.
Relative error (RE) was within 0.24% with corresponding standard deviation within 0.004 for three different determinations (Table 3).
|
Sample No |
Concentration (μg/mL) |
Concentration Found (μg/mL) |
Recovery (% ± SD) |
Relative Error (%) |
|
1 |
5 |
4.7 |
95.5±0.004 |
0.224 |
|
2 |
20 |
19.29 |
96.46±0.002 |
0.15 |
|
3 |
30 |
29.25 |
97.50±0.003 |
0.24 |
Table 3 Evaluation of accuracy and precision
Robustness
Reaction mechanism: It has been reported that NBD-Cl reacts with amino group of primary or secondary amine derivatives.26-29 Similarly, amino group of thiamine can act as a nucleophile due to the lone pair of electrons on the nitrogen atom, trending to attack on the electron–deficient center in NBD-Cl (Table 4 & Figure 6). At the same time, it has been proved that the composition of the product is 1:1 of thiamine and NBD-Cl (Figure 7). So it is concluded that amino group of thiamine react with NBD-Cl to form a brown adduct. The reaction equation is shown in Figure 7.

Figure 6 The Job’s method plot for the stoichiometry of the reaction of Thiamine with NBD-Cl Vr: Volume of NBD-Cl (7.5×10-4 mol/L), Vt: Volume of Thiamine (7.5×10-4 mol/L), Vr + Vt=10 ml.
|
Parameter |
Recovery (% ± SD) |
|
Recommended condition |
97.50±0.003 |
|
NBD-Cl concentration (0.22%) |
98.75±0.002 |
|
NBD-Cl concentration (0.180%) |
96.76%±0.003 |
|
Buffer PH(10.7) |
96.90±0.002 |
|
Buffer PH(10.3) |
95.79±0.22 |
|
Reaction Time min (23) |
96.09±0.009 |
|
Reaction Time min (27) |
98.01±0.003 |
Table 4 Influence of small variation in the assay condition on the analytical performance of the proposed spectrophotometric method for determination of Thiamine using NBD-Cl reagent
Application of the proposed method to analysis of thiamine dosage form
Thiamine tablets were subjected to the analysis by the proposed and the label claim agrees well with our new method as shown in Table 5. The proposed method has the advantage of being virtually free from interferences by excipients.
|
Brand Name of Label Claim (Mg) |
Amount Found (Mg) |
(% Found ± SD)a |
|
Thiamine tablets (100 mg) |
99.96 |
99.9±0.025 |
Table 5 Analysis of Thiamine-containing dosage form by the proposed method
a: values are mean of five determinations.
The present paper described the evaluation of NBD-Cl as analytical reagents in the development of simple, sensitive, and accurate spectrophotometric method for the determination of thiamine in pharmaceutical formulations. The proposed method is simple, reliable, specific, accurate, reproducible, and highly sensitive, for the determination of thiamine in commercially available dosage forms. The procedure presented here does not need necessitate any expensive apparatus; and can be used advantageously as a routine method for the determination of thiamine in quality control labs and industry. The method may be applied to the determination of other secondary amine derivatives as well.
None.
Authors declare that there is no conflict of interest.

©2016 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.