Journal of
eISSN: 2473-0831


Research Article Volume 6 Issue 1
Correspondence: Mohd Dasuki Sul’ain, Department of Biomedicine, School of Health Science, University of Science Malaysia, Health Campus, Kubang Kerian, 15200 Kubang Kerian, Kelantan, Malaysia, Tel 6097677581
Received: July 23, 2017 | Published: September 11, 2017
Citation: Sisin NNT, Abdullah H, Sul’ain MD (2017) Antiproliferative, Antioxidative and Compounds Identification from Methanolic Extract of Passiflora Foetida and Its Fractions. J Anal Pharm Res 6(1): 00166. DOI: 10.15406/japlr.2017.06.00166
Objective: Passiflora foetida is a species of passion flowers and known to have prominent medicinal plant with major phyto-constituents. It belongs to family Passifloraceae that has been widely used in traditional practice for asthma, nervous disorders and hypertensive. This study was conducted to determine the antiproliferative and antioxidant activities of Passiflora foetida leaves methanolic extract (PFME) against MCF-7, MDA-MB-231, HeLa and Hep G2 cancer cell lines, as well as to determine the antiproliferative activity of the major fractions against HeLa cells, and evaluate the compounds present in the PFME and fractions.
Design: The methanolic extract of Passiflora foetida leaves and its fraction were screened for their antiproliferative activities, antioxidant activities and compounds present.
Materials and Methods:Antioxidant activity was evaluated using DPPH assay, FRAP assay, TPC assay and TFC assay. Antiproliferative activity towards MCF-7 (HTB22™) and MDA-MB-231 (HTB-26™) breast cancer cell lines, Hep G2 (HB8065™) liver cancer cell line, HeLa (CCL-2™) cervical cancer cell line and L929 (CCL-1™) normal cell line were observed and compared to tamoxifen as a positive control.
Results:Results indicated that the PFME exhibits a high antiproliferative activity against HeLa (IC50 of 10.83±3.65µg/ml). The PFME manifested high antiradical properties (EC50 of 1.37±1.17µg/ml) and moderate antioxidant reducing power (0.41±0.03mM FE), with 82.09±13.82mg GAE/g of TPC and 205.59±6.57mg QE/g of TFC values. However, fractions shows higher IC50 values against HeLa compared to PFME. GC-MS study revealed the presence 8-32 compounds from each sample. Tetradecanal was identified to possibly possess high anticancer properties.
Conclusion: This study is the first to demonstrate that PF has antiproliferative effect on human cervical cancer cells. PFME might be more potent than the fractions and demonstrates a moderate amount of antioxidant activity.
Keywords: antiproliferative, antioxidant, gc-ms, passiflora foetida, tetradecanal; hela
PFME, passiflora foetida leaves methanolic extract; CCL, cancer cell line; CAM, complementary and alternative medicine; DMSO, dimethyl sulfoxide; CO2, carbon dioxide; FE, ferrous equivalents; Na2Co3,sodium carbonate; GAE, gallic acid equivalent; QE, quercetin equivalents; ANOVA, analysis of variance; SD, standard deviation; DTBP, di-Tert-butylphenol
Each year, approximately 8 million people died from cancers and half of them are in the age of 30 to 69 years old.1 Cancer cases, such as melanoma, prostate, liver, lung and breast cancers, are expected to increase per year in 2010 to 2020.2 The estimated global population of 8.3 billion can be expected by 2025, and thus increases the predicted new cancer cases of 20 million by the same year.3
Among the standard chemotherapeutic drugs that are currently being used in cancer treatments are tamoxifen, doxorubicin and cisplatin. Tamoxifen is an anti estrogen drug that is widely used in the treatment breast cancer and possibly can be used to treat liver and cervical cancers.4,5 Doxorubicin and cisplatin are also used to treat several types of cancer such as liver, bladder, lung, gastric, cervical, colon, and brain cancers.6
Treatments using complementary and alternative medicine (CAM) for cancer are only considered as the additional treatment, not yet as the standard treatments of cancers.7 Problems arose when chemotherapy caused several adverse effects such as low blood cell count, ototoxicity, peripheral neuropathy, menstrual irregularities, fertility disturbance and development of other type of cancer.6 The cancer patients are also gradually developing resistance to the standard chemotherapeutic drugs (Umthong et al. 2011). Thus, it is important to find new anticancer agents. The extracts derived from the natural plants are of interest as the new chemotherapeutic agent sources.8 Since attainment of data on phytochemicals and constituents of medicinal plants in treating and preventing diseases and cancers is significant, it is very important to discern and identify the active constituents in order to develop new natural-based drugs or medicine.
Passiflora foetidaplant was chosen based on its traditional uses for treatment of tumors.9 Extracts of P. foetida had been verified to have anticancer activities on in vivo and in vitro.10,11 The present study is a report of cytotoxic investigation of P. foetida extract and fractions against cervical cancer cell line (HeLa), and the compounds which are possibly responsible for the antiproliferative activity which led to identification of tetradecanal and cis-11-hexadecenal.
Plant materials
PF whole plant was collected from Kelantan, Malaysia. The taxonomic identity of the plant was authenticated by Dr. Shamsul Khamis and was deposited in Herbarium Kulliyyah of Pharmacy, International Islamic University Malaysia (Voucher specimen number PIIUM 0270).
Extraction
The collected PF leaves were rinsed, dried in incubator of 50°C for 3 to 4 days, and grind into powder. A fifty gram of PF dried leaves powder was soaked in 400ml methanol. It was left at room temperature (RT) overnight with occasional stirring. The mixture was filtered using filter paper (240mm) into one container. The filtrate was concentrated by rotary vacuum evaporator and later was air-dried in fume hood for several days. It was referred as PF methanolic extract (PFME).
Antiproliferative study
Collection of cell lines from American Type Culture Collection (ATCC®) were used; MCF-7 (HTB22™) and MDA-MB-231 (HTB-26™) breast cancer cell lines, Hep G2 (HB 8065™) liver cancer cell line, HeLa (CCL-2™) cervical cancer cell line and L929 (CCL-1™) normal cell line. All the cells were maintained by routine passage every two to three days, except for MDA-MB-231 cells which needed to be wash every 24 hours to ensure it continuation to grow well and free from contamination. The method was conducted according to Zakariaet al. (2009) with slight modification. A culture flask with 80-90 % cells confluence was chosen and the detached cells were seeded at 5×104 live cells/ml in a 96-wells plate. When the cells reached 80-90% cells confluence, the old media was discarded. 200µl fresh culture media was added to each well. The PFMEand tamoxifen (positive control) were dissolved in DMSO to make 10mg/ml stock solution. Two-fold serial dilutions were prepared for both PFME and tamoxifen, ranging from 0.39 to 100µg/ml. Each well received 2µl of each concentration from the serial dilution. Negative control cultures received the same concentration of DMSO alone. The 96-well plate was incubated for 72 hours at 37°C incubator in a humidified atmosphere with 5 % CO2. At the end of the incubation period, the old media was discarded and 50µl of 2 mg/ml MTT solution was added to each well. The plate was then incubated again for 4 hours. Then, the MTT solution was replaced with 200μL DMSO. After 20 minutes, absorbance at 570nm was read on a spectrophotometric plate reader. The proportion of surviving cells was/were calculated as ([absorbance of sample/ absorbance of control] x 100). Dose-response curves were constructed to obtain the IC50 values. All experimental data were derived from at least 3 independent experiments.
Fractionation
10 g of PFME was taken up in 234g silica gel 70-230 mesh and run in a fritted 50cm x 40mm column. The extract was eluted with petroleum ether: ethyl acetate (4:1). The type of petroleum ether used was petroleum ether with boiling range of 40-60°C. 10ml to 15ml fractions were collected in test tubes and tested with thin layer chromatography. Fractions with similar spot patterns (same retention factor value) were combined and left to dry.
Antioxidant studies
DPPH assay:According to12 the assay was carried out in a 96-well micro plate with a slight modification. The sample extract was diluted in methanol to prepared 100µg/ml concentration of solution and followed by two-fold serial dilutions. The sample was added to freshly prepared 0.1mM DPPH solution with the ratio of 150µl of DPPH solution to 150µl sample and standard solutions and incubated in dark for 30min at RT. After incubation, the absorbance of the mixture was measured at 517nm against methanol as blank. Gallic acid was used as a positive control,13 as it was found to have the highest antioxidant power and antiradical activity than other compounds.14 Then, scavenging activity (Q) will be calculated as ([Ac–As]/ Ac x 100). Ac was the absorbance of control (DPPH solution without sample) at 517, and As was the absorbance at 517 of sample at different concentrations with DPPH. The antioxidant activity was expressed as EC50 (µg/ml), the concentrations of the sample required to cause a 50% decrease of the absorbance at 517nm. All samples were analyzed in triplicates.
FRAP assay: The method follows closely that used by13 with slight modification. 20μL of 1000µg/ml extract in methanol was mixed with 180μL FRAP reagent in wells of a 96-well plate. The plate was incubated for 6 minutes, and the absorbance was measured at 593nm. FRAP reagent was prepared according to García-Carrasco et al.15 Blanks of sample and solvent only were run together with gallic acid (in MeOH) as a positive control. Iron (III) sulphate (FeSO4) was used as a standard and the concentrations to make a standard curve were 0.1-1.0mM. FRAP activity was calculated as mM Ferrous Equivalents (FE), the concentration of extract/gallic acid which produced an absorbance value equal to that of 1 mM FeSO4. All samples were analyzed in triplicates.
TPC assay: According to Herald et al.16 the TPC assay was carried out in a 96-well microplate with a slight modification. To develop a standard calibration curve, 75µl distilled water, 25µl of gallic acid solutions (0.49 to 250µg/ml), and 25µl of Folin-Ciocalteu reagent (diluted 1:1 (v/v) with dH2O) were mixed together and left for 6 minutes. After that, 100µl of 75g/l sodium carbonate (Na2CO3) solution was added and left in dark for 90 minutes. 1000µg/ml extract was oxidized with Folin-Ciocalteu reagent and neutralized by Na2CO3 solution as given for the standard. The absorbance of solution was recorded at 650nm17 against methanol as blank (set to shake for 60 s before reading). TPC was expressed as mg of gallic acid equivalent (GAE)/g of dry plant material weight (dw). All samples were analyzed in triplicates.
TFC assay: The method follows closely that used by Chatatikun et al.18 with slight modification. Briefly, 50µl of 1 mg/ml extract or standard solution of quercetin (6.25 to 100µg/ml) in 80% ethanol was added to 10µl of aluminium chloride (0.1g/ml) solution and followed by 150µl of 95 % ethanol. 10µl of sodium acetate (83.03g/l) was added to the mixture in a 96 wells plate. 80% ethanol was used as the blank. All mixture were shaken and incubated at RT in dark for 40 minutes. The absorbance was measured at 415nm. TFC was expressed as mg quercetin equivalents (QE)/g dw. All samples were analyzed in triplicates.
Compounds screening using gas chromatography-mass spectrometry
GC-MS analysis was performed on a GC/MS Perkin Elmer ClarusTM SQ8 fitted with an Elite-5MS capillary column (30m L×0.25mm I.D.×0.25μm film thickness). Purified helium (99.999%) was used as carrier gas at a constant flow of 1ml/min. All data were obtained by collecting the full-scan mass spectra within the scan range of 40-500m/z. The sample was prepared in dichloromethane, and the injected sample volume was 1.0μl with a split ratio of 60:1. The oven temperature program was 50°C and accelerated to 120°C at a rate of 10°C/minute, up to 280°C a rate of 5°C/minute. The unknown compounds were identified by comparing the spectra obtained with National Institute Standard and Technology Mass Spectral Library.
Statistical analysis
All statistical analysis was performed using Microsoft Excel and GraphPad PRISM. Each experiment was carried out in triplicate and the results were presented as mean values ± standard deviation (SD) of three independent experiments. The statistical significance of data of the assays obtained were calculated using Student’s t-test, one-way analysis of variance (ANOVA) with Dunnet’s multiple comparison test, and Pearson’s correlation test.
Antiproliferative studies of PFME and fractions on selected cancer cell lines
The IC50 values of PFME were more than 100µg/ml for all the cancer cell lines except for cervical cancer cell line, HeLa. The PFME exhibits a promising anti proliferative activity against HeLa with IC50 value 10.83±3.65µg/ml (Figure 1).
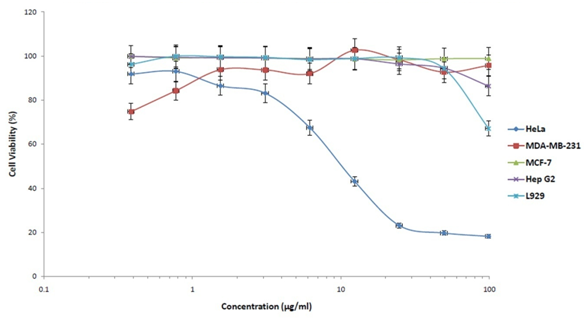
The cell lines are HeLa (cervical), MDA-MB-231 and MCF-7 (breast), Hep G2 (liver) and L929 (normal) cell lines. Each point shows the percentage of viable cells compared to negative control, DMSO.
Figure 1 Antiproliferative activity of PFME against 5 different cell lines by MTT assays.
According to NCI, samples with IC50 value less than 30µg/ml on cancer cell lines could be considered as prospective candidates of new anticancer agents19 and might contain active compounds with anticancer properties.8 Results of the current study were potent enough and fit the characters. Biostatiscally, the mean difference of IC50 values between PFME and positive control drug, tamoxifen, on HeLa is not statistically significant (p > 0.05) (Table 1).
|
Cell Lines |
IC50 (µg/ml) |
||
|
PFME |
Tamoxifen |
||
|
Cancer cells |
HeLa (Cervix) |
10.83±3.65 |
3.08±0.58 |
|
MDA-MB-231 (Breast) |
> 100 |
4.00±0.79 |
|
|
MCF-7 (Breast) |
> 100 |
6.42±1.78 |
|
|
Hep G2 (Liver) |
> 100 |
3.95±0.28 |
|
|
Normal cells |
L929 |
> 100 |
4.00±0.35 |
Table 1 The IC50 values of PFME and tamoxifen against cancer and normal cell lines
The values are represented as mean ± SD.
This may suggest that PFME is as effective as positive control drug on cervical cancer cells. Furthermore, PFME showed low inhibition effects against L929 normal cells, and MDA-MB-231, MCF-7 as well as Hep G2 cancer cells with IC50 values of more than 100µg/ml. If the IC50 values of samples were more than 100µg/ml, it was considered as exhibiting no cytotoxic ability.8,20 Poor cytotoxic activity of sample against normal cells signified its good selective antiproliferative activity (Umthong et al. 2011) and it might have properties that could differentiate the phenotypes between normal and cancerous cells.21 Thus, PFME is considered as cytoselective on cervical cancer cell line.
Since PFME gave the lowest IC50 value on HeLa cell line, thus this cell line was used in the subsequent experiments, for the treatment using the fractions. Table 2 listed the IC50 values of F1 to F5 fractions against HeLa cell line. All fractions showed IC50 values of more than 30μg/ml. Among the five fractions obtained, F5 gave the lowest IC50 value. Even though the value was higher than the NCI reference value, plant extracts with IC50 values within range of 30 to 200μg/ml have the potential to be refined into anticancer agents19,22 Based on the overall evaluations of antiproliferative activities among PFME and its fractions, the IC50 value of F5 was higher than the PFME. The findings suggested that the PFME was more potent than the fractions. It is possible that combinations of several compounds from different fractions might generate synergistic effect on antiproliferative activity.23
|
Positive Control and Fractions |
IC50 (µg/ml) |
|
|
Tamoxifen |
2.90 ± 0.21 |
|
|
PFME Fractions |
F1 |
>100.0 |
|
F2* |
65.67±2.08 |
|
|
F3* |
80.00±5.20 |
|
|
F4* |
66.50±3.04 |
|
|
F5* |
47.83±7.59 |
|
Table 2 The IC50 values of tamoxifen and five fractions against the HeLa cell line
The values are represented as mean ± SD. * Mean difference was significant (p < 0.05) against positive control, tamoxifen.
Antioxidant studies of passiflora foetida methanolic extract (PFME)
Since PFME was found to be more potent in antiproliferation of cancer cells, only PFME was tested for its antioxidant activity. The estimated EC50 value of PFME was 1.37±1.17µg/ml, which was higher than the positive control gallic acid (Table 3). High free radical scavenging activity of a sample was indicated by its low EC50 value.24,25 The present finding may suggest that PFME have high antiradical abilities. This hypothesis is in line with other previous studies. The extract might be have active antioxidant compounds if its EC50 value was less than 4000µg/ml,17 and it might has extremely high antiradical activity if its EC50 value was less than 10µg/ml.26
|
DPPH |
FRAP |
TPC |
TFC |
|
|
EC50 (µg/ml) |
(mM FE) |
(mg GAE/g) |
(mg QE/g) |
|
|
PFME |
1.37±1.17 |
0.41±0.03a,b |
82.09±13.82a |
205.59±6.57a |
|
Gallic acid |
< 0.39 |
2.26±0.04 |
- |
- |
Table 3 Antioxidant activity of PFME determined by DPPH, FRAP, TPC and TFC methods
Experiments were performed in triplicate and expressed as mean ± SD. a Concentration used was 1000µg/ml. b The mean difference was significant (p < 0.05) against positive control, gallic acid.
The DPPH radical scavenging activity of PFME was also expressed to have positive correlation with FRAP results (R=0.957). FRAP assay demonstrated that PFME contained moderate amount of antioxidative compounds (0.41±0.03mM FE). However, in comparison to the positive control gallic acid, the PFME was significantly less active, biostatistically. Extracts with high antioxidative activity have FRAP value approximately more than 0.9mM, while extracts with low antioxidative activity have FRAP value approximately less than 0.011mM.27 Thus, gallic acid was confirmed to be highly antioxidative, and PFME was only moderately antioxidative.
Furthermore, in TPC assay, a linear calibration curve of gallic acid with GAE equation y = 0.005x + 0.083 (r2 = 0.996) was obtained. The presence of phenols in PFME was measured up to 82.09±13.82mg GAE/g. Extracts with TPC value of more than 20mg GAE/ g was considered as a high value.28 This hypothesis contradicts with another study12 who coordinated range groups of TPC values. High TPC of samples should be within the values of more than 400mg GAE/g, while moderate TPC group is within the values of 150-400mg GAE/g. However, PFME fell into the last group; the low TPC with value of less than 150mg GAE/g. Similarly, the current finding was supported by another two studies29,30 that found low amount of TPC in PF leaves.
The TPF value was also demostrated to have positive correlation with TFC (R = 0.783). The TFC of PFME quantified was 205.59±6.57mg QE/g, using QE equation y = 0.000374x+0.024 (r2 = 0.999). The present study demonstrated that PFME had moderate TFC. Extracts with moderate TFC exhibited values within the range of 100-800mg QE/ g.31 However, the current findings contradicts to a study30 which found that the TFC of the PF leaves extract was low. This may be due to the difference of solvent and extraction methods used for extraction.
Many studies have proven that extracts with antioxidant effects were also reported to have anticancer properties.29,32-35 This statement is in agreement with the current findings. Antioxidant could protect the normal cells from DNA damage and restrain cancer cells development36 and might cause antiproliferation of cancer cells by affecting antioxidant enzymes37 and apoptosis in the cancer cells.32
Compounds screening using GC-MS
From the GC-MS analysis, a total of 8 compounds were identified in PFME, 16 compounds were identified in each F1 and F2, 18 compounds were identified in F3, 25 compounds were identified in F4, and 31 compounds were identified in F5. These compounds were resolved at concentrations ranging from 0.19 to 48.83 per cent, and retention time ranging from 13.56 to 39.92minutes. Primarily, discussions will be focussed on the eight compounds detected from PFME and their association to compounds identified in F1 to F5 fractions (Table 4).
|
No. |
Compounds |
Percent Area (%) |
|||||
|
PFME |
F1 |
F2 |
F3 |
F4 |
F5 |
||
|
1 |
10-octadecenal |
12.63 |
- |
1.38 |
- |
0.66 |
- |
|
2 |
13-heptadecyn-1-ol |
- |
- |
- |
- |
1.5 |
- |
|
3 |
15-octadecenal |
- |
- |
- |
0.83 |
- |
0.4 |
|
4 |
16-octadecenal |
- |
- |
- |
- |
- |
0.56 |
|
5 |
17-pentatriacontene |
- |
- |
- |
0.36 |
0.94 |
0.57 |
|
6 |
1-aminoinosine |
- |
- |
- |
- |
0.29 |
- |
|
7 |
1-eicosanol |
- |
- |
- |
- |
- |
4.55 |
|
8 |
1-heptatriacotanol |
- |
1.36 |
7.46 |
12.6 |
3.74 |
- |
|
9 |
1-hexacosene |
- |
- |
- |
- |
1.44 |
- |
|
10 |
1-hexadecanol |
- |
- |
- |
- |
- |
0.73 |
|
11 |
2,4-di-tert-butylphenol |
- |
- |
- |
- |
0.93 |
0.68 |
|
12 |
2-bromooctadecanal |
- |
- |
- |
- |
- |
0.29 |
|
13 |
2-cis-9-octadecenyloxyethanol |
- |
- |
2.39 |
8.45 |
1.05 |
1.58 |
|
14 |
2-cyclohexen-1-one-4-ol-3,5,5-trimethyl-4-(3-oxo-1-butenyl) |
- |
- |
- |
- |
- |
9.35 |
|
15 |
2-methylenecholestan-3-ol |
- |
- |
14.66 |
- |
- |
- |
|
16 |
2-methylhexadecan-1-ol |
- |
1.1 |
1.1 |
- |
- |
- |
|
17 |
3-(benzylthio)acrylic acid methyl ester |
- |
9.15 |
- |
- |
- |
- |
|
18 |
3,5-di-tert-butylphenol |
5.95 |
- |
- |
- |
- |
- |
|
19 |
3-eicosyne |
- |
3.66 |
- |
- |
- |
- |
|
20 |
3-ethyl-5-(2-ethylbutyl)octadecane |
- |
- |
- |
- |
- |
0.66 |
|
21 |
5-octadecenal |
5.85 |
- |
- |
- |
- |
- |
|
22 |
5-pentylresorcinol |
- |
- |
- |
- |
2.16 |
0.48 |
|
23 |
7-hexadecenal |
- |
- |
- |
3.73 |
- |
- |
|
24 |
7-hexylicosane |
- |
- |
- |
- |
- |
0.75 |
|
25 |
Behenic alcohol / 1-docosanol |
- |
- |
- |
- |
- |
0.74 |
|
26 |
Bis (2-ethyl hexyl) maleate |
- |
- |
- |
- |
- |
12.37 |
|
27 |
Bis (2-ethyl hexyl) phthalate |
- |
- |
- |
- |
- |
7.8 |
|
28 |
Butyl-9,12,15-octadecatrienoate |
- |
- |
- |
14.48 |
- |
- |
|
29 |
Butyloctylphthalate |
- |
- |
- |
- |
- |
1.14 |
|
30 |
Cis-11-hexadecenal |
48.2 |
- |
- |
- |
48.83 |
- |
|
31 |
Cis-13-eicosenoic acid |
- |
- |
- |
- |
0.29 |
- |
|
32 |
Cis-13-octadecenal |
- |
- |
- |
- |
0.19 |
- |
|
33 |
Cis-9-hexadecenal |
- |
- |
- |
- |
- |
4.25 |
|
34 |
Dibromotetrapentacontane |
- |
- |
- |
- |
- |
1.43 |
|
35 |
Diepoxyhexadecane |
- |
- |
1.89 |
- |
- |
|
|
36 |
Diethyleneglycol monododecyl ether |
- |
2.72 |
- |
- |
- |
- |
|
37 |
Dihydroactiridiolide |
- |
- |
- |
1 |
1.82 |
- |
|
38 |
Dihydroagarofuran |
- |
- |
- |
- |
0.85 |
- |
|
39 |
Dihydroxycholecalciferol/dihydro vitamin D |
- |
1.8 |
- |
- |
- |
- |
|
40 |
Dioctyl adipate |
- |
- |
- |
- |
- |
2.97 |
|
41 |
Dioctyl isophthalate |
- |
- |
- |
- |
- |
28.52 |
|
42 |
Dipalmitin |
- |
- |
6.65 |
0.93 |
- |
0.62 |
|
43 |
Dodecanal |
- |
- |
- |
- |
1.88 |
1.17 |
|
44 |
Dodecyl hexaethylene glycol |
- |
11.36 |
- |
- |
- |
- |
|
45 |
E-3-pentadecen-2-ol |
- |
- |
- |
- |
0.26 |
- |
|
46 |
Eicosatrienoic acid methyl ester |
12.25 |
- |
- |
- |
- |
- |
|
47 |
Erucic acid |
- |
- |
4.57 |
- |
0.27 |
- |
|
48 |
Ethyl iso-allocholate |
3.38 |
- |
2.51 |
18.74 |
- |
1.28 |
|
49 |
Heptacosane |
- |
- |
- |
- |
- |
0.25 |
|
50 |
Heptaethylene glycol monododecyl ether |
- |
7.95 |
- |
- |
- |
- |
|
51 |
Hexadecanoic acid mono(2-ethylhexyl)ester |
- |
1.69 |
- |
- |
- |
- |
|
52 |
Hexadecenal |
- |
- |
- |
- |
17.85 |
4.78 |
|
53 |
Hexadecyl trichloroacetate |
- |
- |
- |
- |
|
|
|
54 |
Hexahydro farnesyl acetone |
- |
- |
26.94 |
11.76 |
4.07 |
2.32 |
|
55 |
Isophytol |
- |
- |
1.07 |
- |
- |
- |
|
56 |
Lauryl triethoxylate |
- |
8.98 |
- |
- |
- |
- |
|
57 |
Linoleic acid athyl ester |
- |
- |
- |
- |
0.51 |
- |
|
58 |
Linoleoyl chloride |
- |
- |
- |
- |
0.64 |
- |
|
59 |
Methyl hexadecanoate/ Hexadecanoic acid methyl ester |
- |
6.99 |
3.85 |
- |
- |
3.16 |
|
60 |
Methyl-8-tetradecenyl acetate |
- |
- |
- |
10.42 |
- |
- |
|
61 |
Nonadecatriene-5,14-diol |
- |
- |
- |
- |
2.67 |
- |
|
62 |
Octadecadienoic acid methyl ester |
- |
- |
2.53 |
- |
- |
- |
|
63 |
Octadecanal |
- |
- |
1.39 |
0.41 |
- |
- |
|
64 |
Octaethylene glycol monododecyl ether |
- |
13.76 |
- |
- |
- |
- |
|
65 |
Octatriacontyl pentafluoropropionate |
- |
- |
- |
- |
0.71 |
- |
|
66 |
Phytol |
- |
- |
9.71 |
3.14 |
- |
- |
|
67 |
Phytol acetate |
14.04 |
11.41 |
2.81 |
5.25 |
- |
|
|
68 |
P-methan-1-ol |
- |
- |
- |
- |
0.19 |
- |
|
69 |
Sacreroside |
- |
3.3 |
- |
- |
- |
- |
|
70 |
Tert-hexadecanethanol |
- |
- |
5.77 |
- |
- |
- |
|
71 |
Tert-hexadecanethiol |
- |
- |
- |
- |
- |
3.47 |
|
72 |
Tertramethyl-2-hexadecene-1-ol |
- |
2.76 |
9.4 |
8.19 |
- |
- |
|
73 |
Tetradecanal |
17.69 |
- |
- |
- |
- |
- |
|
74 |
Tetradecyl trichloroacetate |
- |
- |
- |
- |
- |
1.83 |
|
75 |
Tetratetracontane |
- |
- |
- |
- |
- |
0.27 |
|
76 |
Trimethyltetradecane |
- |
- |
- |
- |
- |
1.03 |
|
77 |
α-glyceryl linolenate |
- |
- |
- |
10.42 |
- |
- |
|
78 |
γ-palmitolactone |
- |
7.05 |
- |
- |
- |
- |
Table 4 Comparative GC-MS analysis of compounds in PFME and fractions (F1 to F5). 8 to 32 compounds were identified in the PFME and the fractions. Overall, a total of 88 compounds detected in GC-MS spectra of the six samples
Biologically important compounds that were determined from PFME were cis-11-hexadecenal (48.20 %), tetradecanal (17.69%), phytol acetate (14.04%), 3, 5-di-tert-butylphenol (5.95%), 5-octadecenal (5.85), ethyl iso-allocholate (3.38%), 10-octadecenal (2.63%), and eicosatrienoic acid methyl ester (2.25%) (Figure 2).Nonetheless, no previous data had recorded the anticancer activity of extracts that contained cis-11-hexadecenal and 10-octadecenal. Although cis-11-hexadecenal is the most abundant compound in PFME and F4, there were scarce reports on the bioactivity of the compounds, except its associations as an antidiabetic component from P. foetidaplant39 and an insect sex pheromones agent.39 Besides, the only bioactivity of extracts with 10-octadecenal reported was antibacterials.40 Hence, this present study is the first to address the possible relationship between cis-11-hexadecenal and 10-octadecenal with the in vitro antiproliferative effect on HeLa cancer cells, which might occurred in synergism as the effect of PFME or buffering effect as of F4 with other compounds.
Second major compound identified in PFME was tetradecanal. Previously, this aldehyde compound was discovered in fractions of Sarcopoterium spinosum.41 In agreement to the present study that testified the antiproliferative effect of PFME on HeLa cell line, the fractions of S. spinosum was found to have IC50 value of 32.1μg/ml against HeLa cell line.41 As tetradecanal was present in PFME and absent in all fractions, it might justify that tetradecanal could have high potential antiproliferative effect on HeLa cells.
Additionally, phytol acetate is the third major compound in PFME. A previous report on Dicranumscopariumproposed that fractions of the plant contained phytol acetate and proffered antiproliferative effects on HeLa cancer cells.42 Since phytol acetate was also present in F1, F3 and F4, the findings might indicate that phytol acetate did not have high impact on antiproliferation of cancer cells.
Another biologically important compound in PFME was 3,5-di-tert-butylphenol (3,5-DTBP), a phenol derivative. The extract containing 3,5-DTBP was proclaimed to cause DNA damage and cytotoxicity towards Hep G2 cancer cell line43). Its isomer 2,4-di-tert-butylphenol (2,4-DTBP) was identified in F4 and F5 fractions. Isomerization is one of the major decomposition processes that could occur during fractionation.44 The 2,4-DTBP was found in a fraction of Pereskia bleo leaves. The extract possessed very remarkable cytotoxic activity against MCF-7 with IC50 value of 5.75µg/ml.45 However, PFME was not cytotoxic on Hep G2 and MCF-7 as reported by the above studies. Thus, it is assumed that both phenol derivatives are not critical in anticancer process.
Meanwhile, ethyl iso-allocholate was detected in PFME as well as F2, F3 and F5. The extracts of other plants that comprised ethyl iso-allocholate compound have also showed antiproliferative activities against MCF-7 and Hep G2 with IC50 values within the range of 31.08 to 78.89µg/ml.34,46,47 The current data contradicts to the mentioned studies as PFME showed no antiproliferative effects on MCF-7 and Hep G2 cancer cells. Additionally, ethyl iso-allocholate was the most abundant compound in F3, yet it caused low cytotoxicity against HeLa cells. This situation may denote that ethyl iso-allocholate not selective towards MCF and Hep G2 cells and could cause buffering effects.
Lastly, eicosatrienoic acid methyl ester had also been identified in PFME. This compound had also been detected in a fraction of Echeveria subrigida plant, with 97.6 % inhibition of mutagen1-NP on SalmonellaTyphimurium YG1024 using Kado microsuspension assay.48 Gene mutation caused development of cancer,26 thus antimutagen could be considered as anticancer. Therefore, eicosatrienoic acid methyl ester might have potential compounds could be a candidate of anticancer agents.
In relation to the antioxidant study of PFME, there is no record on antioxidant activity of cis-11-hexadecenal. The moderate antioxidant activity of PFME might be the attributes of the minor compounds; 3,5-DTBP,49 eicosatrienoic acid methyl ester,50 ethyl iso-allocholate,34 phytol acetate,51 and tetradecanal.52
In conclusion, PFME exhibit more potent antiproliferative activity compare to fractions. The PF extract demonstrated a high antiproliferative effect on human cervical cancer cells. The effect could be contributed by tetradecanal compound. Cis-11-hexadecenal and 10-octadecenal could also have the potential to cause in vitro antiproliferative effect towards cervical cancer cells. Individual or combinations of several compounds might have synergistic or buffering effects on antiproliferative and antioxidant activities.
The authors are grateful to Kulliyyah of Pharmacy and Kuliyyah of Science, International Islamic University Malaysia for providing necessary facilities (herbarium and GC-MS services) to carry out this work successfully

©2017 Sisin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.