Journal of
eISSN: 2473-0831


Research Article Volume 8 Issue 3
1Department of Zoology, Ranchi University, India
2Vice Chancellor, Sidho Kanhu Murmu University, India
Correspondence: Sukumar Dandapat, Department of Zoology, Ranchi University, Ranchi-834008, Jharkhand, India
Received: February 24, 2019 | Published: May 1, 2019
Citation: Jose S, Dandapat S, Sinha MP. Anti-hypertensive activity of aqueous and methanolic leaf extracts of Schleichera oleosa (Lour.) Merr. J Anal Pharm Res.2019;8(3):94?97. DOI: 10.15406/japlr.2019.08.00320
Present study was undertaken to investigate phytochemical, nitric oxide scavenging activity and antihypertensive activity of the methanolic and aqueous leaf extracts of S. oleosa. The phytochemical analysis carried out revealed the presence of flavanoids, glycosides, alkaloids, tannins, saponins and steroids and many other metabolites. Both the extracts showed high NOSA (33.81% and 31.43% NOSA at 100µg concentration). The methanolic leaf extract showed 1.6%, 4%, and 5.6% ACE inhibition against 10µg, 50µg and 100µg concentration respectively and aqueous leaf extract of S. oleosa showed very negligible percentage that is 0%, 0% and 0.8% ACE inhibition against 10µg, 50µg and 100µg extract concentration respectively while Captopril showed 100% ACE inhibition against all the three concentrations. The result shows that the methanolic as well as the aqueous leaf extracts of Schleichera oleosa are not very good antihypertensive agents. However the methanolic leaf extract of Schleichera oleosa is a better antihypertensive inhibitor than the aqueous leaf extract.
Keywords: captopril, hypertensive, phytochemical, radical scavenging
HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; ACE, angiotensin-converting enzyme; ELISA, enzyme-linked immunosorbent assay; NOSA, nitric oxide scavenging activity; ME, methanolic extract; AE, aqueous extract; ROS, reactive oxygen species; NO, nitric oxide; RNS, reactive nitrogen species
Diseases related to heart and circulatory systems are the reasons for 12 million deaths every year globally and are known to be the major group of killer disease.1 Hypertension is the most common problem associated with cardiovascular disease and constituents a major factor for several cardiovascular pathologies including atherosclerosis, coronary artery disease, myocardial infract heart failure, renal insufficiency, stroke and dissecting aneurysm of aorta and is one of the leading causes of disability, mortality and morbidity along the population and it is the most common chronic illness faced in the world specially developing countries.2,3
Because of high incidence and morbidity, various drugs and regimes have been advocated for the control of hypertension. Many new drugs have also been introduced which may demonstrate better efficacy but possess side effects.4 Recently, attention has been focused towards herbal and mineral preparations which are traditionally used as potential therapeutic agents in the prevention and management of cardiovascular diseases and hypertensive agent.5
Genus Schleichera is belonging to the soapberry family Sapindaceae and is generally found in Indian subcontinent and in mixed deciduous forests and in Southeast Asia.6 Schleichera oleosa has been used in traditional medicine and its leaf, seed, oil and bark are used for curing itch, burns, acne pain etc., and it promotes hair growth, treats rheumatism, head ache, skin diseases, malarial fever and is prophylactic against cholera.7
Screening of proximate phytochemicals and in vitro antihypertensive activity of the leaf extracts of S. oleosa has not been explored previously. The present study was undertaken to screen the proximate phytochemical composition and in vitro antihypertensive activities of the aqueous and methanolic leaf extracts of S. oleosa.
Collection of plant material
The fresh mature leaves of the plant were collected and identified. The leaves were washed and dried in shade under room temperature for six to seven days and then crushed into coarse powder using electric grinder. The powder was sieved to get fine powder using fine plastic sieve which was stored in air tight bottle in the laboratory until required.
Extract preparation
50g of the powder was subjected to extraction by soxhlet using methanol (300mL) and distilled water (300mL) separately. The extracts obtained were filtered, concentrated after dryness in rotary flash evaporator maintained at 45ºC. Percentage yield of each extract was calculated and the dried extracts were stored in airtight containers at room temperature for further studies.8
Phytochemical analyses
Freshly prepared extracts of the powdered leaves were subjected to phytochemical analyses to find the presence of the following phyto constituents such as flavanoids, alkaloids, carbohydrates, glycosides, polysaccharides, tannins, saponins, steroids, proteins, lipids, oils by standard methods of Arya et al.,9 and total phenol and flavonoid was estimated by previous methods of Dandapat et al.10
Nitric oxide radical scavenging activity
NOSA was performed as per previous method of Kumar et al.,11 10μg, 50μg and 100μg concentrations of aqueous and methanolic plant extract and Butylated hydroxy anisole (BHA) were taken in different test tubes and made up to 3ml with 0.1M phosphate buffer (pH 7.2). Sodium Nitroprusside (5mM) prepared in buffered saline (pH 7.2) was added (1ml) to each tube and made final sample for nitric oxide radical scavenging test. The reaction mixture (final sample) was incubated for 30 min at RT. A control (methanol) equivalent amount to final sample without the extract was maintained. After 30 min, 1.5ml of above solution was mixed with 1.5ml of Griess reagent (1% Sulphanilamide, 2% phosphoric acid and 0.1% N-1- Naphthylethylenediamine dihydrochloride). The absorbance of the samples was measured at 546nm. Nitric oxide radical scavenging activity was calculated using the following formula:
Estimation of antihypertensive activity
Estimation of antihypertensive activity was performed as per as previous method of Hooper et al.12 Jimsheena et al.,13 10µg, 50µg and 100µg of S. oleosa aqueous and methanolic leaf extract was tested for antihypertensive activity. Plant extracts were dissolved in methanol and mixed with 10mM HEPES assay buffer (0.3M NaCl and 10µM Zinc Sulphate), 20µL kidney cortex plasma membranes (ACE enzyme source) and 1mM Hippuryl-His-Leu as substrate. The mixture was incubated for 10 minutes at 37oC. Then 10µL of substrate (1mM) was added which makes a final reaction volume of 50µL and furter incubated for 45min at 37°C. The reaction is terminated by the addition of 1M HCl (0.1mL). The yellow color is developed by the addition of 100µL of pyridine and 50µL of Benzene sulphonyl chloride. The yellow color that formed is measured at 410nm in an ELISA plate reader (iMARK, BIORAD). Compounds with an inhibitory potential block the substrate availability to the enzyme and thereby cause enzyme inhibition leading to no formation of yellow color. The inhibition is represented in the form of percentage over control. Captopril, a known ACE inhibitor is tested in this assay as a standard compound. The same procedure was used for aqueous extract to analyze for its antihypertensive activity.
Phytochemicla screening
Resuts of proximate qualitative phytochemical screening of S. oleosa methanolic and aqueous extract is presented in Table 1. Results revealed the extracts contain carbohydrates, glycosides, polysaccharides, proteins alkaloids, steroids triterpenes, flavanoids, tannins. Quantitative estimation of total flavonoid and total phenolics are presented in Figure 1. The results of quantitative estimation of total flavonoid and total phenolics content of the extract showed that, both total flavonoid (168.89µg/mg) and total phenolics (20.00µg/mg) content of methanolic extract are high than aqueous leaf extracts of S. oleosa.
Phytochemicals |
ME |
AE |
Carbohydrates |
+ |
+ |
Glycosides |
+ |
+ |
Polysaccharides |
+ |
- |
Proteins |
+ |
+ |
Alkaloids |
+ |
+ |
Steroids |
+ |
+ |
Triterpenes |
+ |
_ |
Flavanoids |
+ |
- |
Tannins |
+ |
- |
Lipid |
_ |
+ |
Oils |
+ |
+ |
Saponins |
- |
+ |
Table 1 Proximate Phytochemical composition of Methanolic and aqueous extracts of S. oleosa
Nitricoxide radical scavenging activity
Results of NOSA of methanolic and aqueous leaf extracts of S. oleosa are present in Figure 2. Results revealed the NOSA of BHA (6.19%, 28.57% and 42.86%) is higher than the extracts but the methanolic (7.14%, 13.33% and 33.81%) extract showed high NOSA than aqueous extract (1.90%, 10.00% and 31.43%).
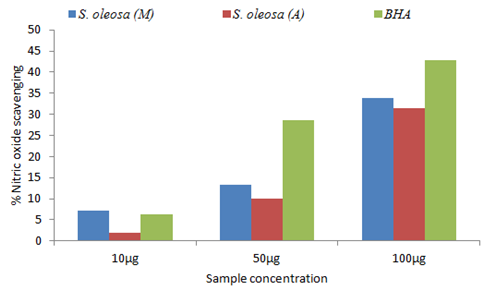
Figure 2 Nitricoxide radical scavenging activity of S. oleosa methanolic (M), aqueous (A) leaf extract and BHA.
Antihypertensive activity
The antihypertensive efficacy of the extracts of S. oleosa leaves was quantitatively assessed on the basis of ACE inhibition percentage and compared with standard ACE inhibitor Captopril. The results of are shown in Table 2. Antihypertensive activity of Captoprill was also tested against the same concentration of extracts to compare antihypertensive efficacy of S. oleosa leaf extracts. The methanolic leaf extract showed 1.6%, 4%, and 5.6% ACE inhibition against 10µg, 50µg and 100µg concentration respectively and aqueous leaf extract of S. oleosa showed very negligible percentage that is 0%, 0% and 0.8 ACE inhibition against 10µg, 50µg and 100µg extract concentration respectively while Captopril showed 100% ACE inhibition against all the three concentrations.
Concentration of leaf extracts |
% Inhibition of ACE |
||
SME |
SAE |
Captoprill |
|
10µg |
1.6 |
0 |
100 |
50µg |
4 |
0 |
100 |
100µg |
5.6 |
0.8 |
100 |
Table 2 Showing the ACE inhibition of S. oleosa methanolic leaf extract, aqueous leaf extract and Captoprill
These constituents are responsible for the curative nature of Schelichera oleosa against itching, head ache, malaria, skin diseases etc. which could make the plant useful for treating different ailments and having a potential of providing useful and safe drugs and drug leads for human use.14 Biochemicals of plants natural substances such as plants, mushrooms and algae etc. posses antioxidants activity which are associated with reduction of free radicals, modulation of immune system, pathogen suppression and health protective activities and many other therapeutic activities.15,16 Previous in vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract and found concentration dependent NOSA which are associated phytochemicals such as phenol, flavonoid, saponins, tannins, vitamins etc.17 Phenolic compounds polyphenolic compounds such as flavonoids, tannins and alkaloids are found in the edible and inedible parts of plants portray antioxidant activity and mainly due to their redox properties, which allows them to act as reducing agents, hydrogen donors and singlet oxygen quenchers or metal chelators18-20 hence they play anti-oxidizing effects reduces the free radical generation. In present study S. oleosa leaf extracts also contain different types of phytochemicals and high phenolic and polyphenolic content which may associate with free radical scavenging and antihypertensive activity and other medicinal properties. Oxidative stress is one of the major key factor that promotes pathogenesis of hypertension in human and other mammals.21 Endothelial cells play a major role in the contraction and relaxation of blood vessels. They release nitric oxide which impart in vasorelaxation and maintain normal blood pressure.22,23 Reactive nitrogen species such as nitric oxide synthase (NOS), and in particular the endothelial isoform of NOS (eNOS), are now recognized as an important source of superoxide anions which combine with other reactive oxygen species (ROS) suppress the production of nitrous oxide (NO) and form nitrosative stress, damage the cells, decrease atherogenic stimuli for NOS coupling and NO production and promote vasoconstriction.24,25 It has also been reported that, eNOS become a peroxynitrite generator of free radicals, leading to a dramatic increase in oxidative stress, since peroxynitrite formed by the NO-superoxide reaction has additional detrimental effects on vascular function by oxidation of cellular proteins and lipids.23,26 It has been reported that reactive nitrogen species (RNS) such as NO2, N3O4, N2O4 etc. are associated with NO-superoxide reaction but reduction in the excess level of NO in human body by taking antioxidant rich plant products can lower the risks of various pathophysiological actions such as elevated inflammation, hypertension, cancer etc and maintained good health status.27 Earlier works on ACE inhibition by plants like Lippia nodiflora have shown positive results.28 The standard chemotherapeutic drugs include direct vasodilation of the blood vessel, blocking of calcium channels, inhibition of α-adrenoreceptor response, induction of negative ionotropic response of smooth muscle, inhibition of platelet aggregation, reduction of vascular resistance, and improvement of pulmonary oxygen utilization.29,30 It has been reported that enhanced activity of nitric oxide and improved handling of intracellular calcium has also been found to play a critical role in the reduction of vascular resistance and blood pressure that are elevated in hypertensive rats and humans.31 The antihypertensive effects of plants are associated with their antioxidant properties because oxidative stress is considered a major risk factor in hypertension and cardiovascular diseases.32 In previous research free radical and nitric oxide radical scavenging activity of five medicinal plants Spatholobus suberectus, Uncaria rhynchophylla, Alpinia officinarum, Drynaria fortunei and Crataegus pinnatifida traditionally used for the treatment of hypertension have been studied and reported these plants contains various kinds of phytochemicals such as phenols and polyphenols, alkaloid etc. effectively reduced free radicals.33 Njoya EM et al.,34 also worked on free radical scavenging activities of Sarcocephalus pobeguinii extracts and reported the extract inhibit formation of free radicals and reduces nitric oxide radicals which are associated with pathogenesis of hyper tension. In present work S. oleosa leaf extract inhibits the nitric oxide radicals and also posse antihypertensive activity which correlates with previous studies.
S. oleosa leaf extract contain various phytochemicals but phenols and polyphenolic phytochemicals are found in high concentration. In vitro nitric oxide radical scavenging activity of the extracts are not high than synthetic antioxidant BHA. Methanolic extract of S. oleosa showed high antihypertensive activity compare to aqueous extract but their antihypertensive activity is quite low compare to standard Captopril. Thus from the present work it can be concluded S. oleosa leaf extract possess antioxidant and antihypertensive properties. Further, more extensive in vivo work on animal model will be required.
The authors acknowledge the facilities provided by Department of Zoology, Ranchi University, Ranchi, India for carrying out the work.
There is no conflict of interest among the authors.

©2019 Jose, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.