Journal of
eISSN: 2473-0831


Research Article Volume 4 Issue 4
Correspondence: Sahil Sharma, Laboratory for Drug Design and Synthesis, Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga-142001, Punjab, India
Received: March 28, 2017 | Published: April 17, 2017
Citation: Sharma S (2017) A New Synthetic Route for the Synthesis of 2-Aryl/Heteroaryl Pyrazolo [1,5- C ] Quinazolines. J Anal Pharm Res 4(4): 00111. DOI: 10.15406/japlr.2017.04.00111
Quinazoline containing compounds have been widely reported in the literature owing to their biological potential such as antimicrobial, anti-leishmanial, antitumour, anti-inflammatory properties. Keeping in view their biological attributes, I herein report a new synthetic route for the synthesis of 2-aryl/heteroaryl pyrazolo [1,5-c] quinzoline derivatives. Successfully synthesized quinazoline derivatives were further characterized by IR, NMR and elemental analysis.
Keywords: quinazoline, aryl/heteroaryl, antimicrobial, anti-leishmanial, antitumour, anti-inflammatory, ir, nmr, pyrazolo, tricyclic, acetophenones, aldehydes, pyrazole, cyclization
singlet; bs, broad singlet; d, doublet; t, triplet; dd, doublet of doublets; m, multiplet; IR, infrared; FT-IR, fourier transform infrared; TLC, thin layer chromatography; UV, ultra violet
Quinazoline containing compounds have been widely reported in the literature owing to their biological potential such as antimicrobial, anti-leishmanial, antitumour, anti-inflammatory properties.1-5 The interest in tricyclic 5:6:6 systems with one ring junction nitrogen atom between the five-membered and six-membered rings, stems from its appearance in many biologically active compounds, for example, thiazolo [4,3-b] quinazolines as antitumour agent and imidazo [1,2-c] quinazoline as anti-inflammatory agents.4,5 Pyrazolo [1,5-c] quinzolines having tricyclic 5:6:6 systems have been reported in the mainstream as well as in the patent literature as one of the biologically active moiety.6-10 Keeping in view the biological attributes pyrazolo [1,5-c] quinzolines, I herein report a new synthetic route for the synthesis of 2-aryl/heteroaryl pyrazolo [1,5-c] quinzoline derivatives.
The synthetic route to the title compounds, 4a-d (Figure 1) is outlined in Scheme 1. The desired compounds were synthesized via sequence of reaction starting from Claisen Schmidt condensation of aryl acetophenones and aryl aldehydes. The method is attractive as it selectively yields E isomer from 1H NMR. All diaryl propenones were found to be geometrically pure and with trans configuration (J=15-16 Hz). 3-(2-Nitrophenyl)-1-arylprop-2-en-1-ones (1a-d) on treatment with hydrazine hydrate in formic acid underwent a transposition reaction with simultaneous formylation to yield 4,5-dihydro-5-(2-nitrophenyl)-3-arylpyrazole-1-carbaldehydes (2a-d). These compounds showed a characteristic singlet in 1H NMR spectra for aldehydic proton along with a classical ABX system displayed by the protons of non aromatic flexible N-formyl pyrrazole ring. Further the reduction of the nitro group was attempted by number of synthetic stratergies (Figure 2). But the reduction was successful with ferrous sulphate/ammonia solution to yield 5-(2-aminophenyl)-4,5-dihydro-3-arylpyrazole-1-carbaldehydes (3a-d) (Scheme 1). In the case of 4a and 4b 5-(2-aminophenyl)-4,5-dihydro-3-arylpyrazole-1-carbaldehydes (3a and 3b) were isolated and were further cyclised undergoing simultaneous dehydrogenation on stirring in methanol to furnish the desired title compound. However in the case of 4c and 4d the intermediates 3c and 3d could not be isolated and the intermediate 2 on reduction underwent a simultaneous cyclization and dehydrogenation. The reason for the simultaneous dehydrogenation in all the desired compounds can be attributed to the thermodynamic stability of the aromatized system (14π electrons) having 4n+2π electrons. The desired compounds were characterized by the appearance of a singlet of the proton of quinazoline moiety.
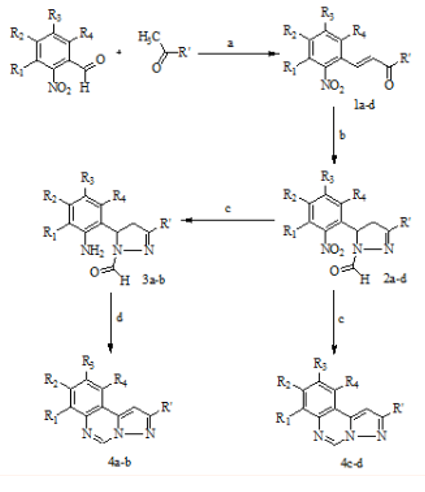
Scheme 1 Reagents and conditions: (a) CH3COOH, H2SO4, stir, rt, 24h; (b) HCOOH, NH2NH2.H2O, reflux, 4h; (c) FeSO4/NH4+ CH3OH, stirr, rt. 2h; (d) CH3OH, stir, rt, 6h.
Experimental Section
General procedures
Melting points were recorded on Decibel Digital melting point apparatus in open glass capillary tubes and are uncorrected. The 1H & 13C NMR spectra were recorded on a Bruker Avance DEX 400 MHz instrument with CDCl3 as solvent at 400 and 100 MHz, respectively. Chemical shifts were reported in δ (ppm) downfield from tetramethylsilane, and coupling constants (J) are expressed in hertz. Multiplicities are recorded as s (singlet), bs (broad singlet), d (doublet), t (triplet), dd (doublet of doublets), m (multiplet). Infrared (IR) spectra of compounds were recorded on Fourier Transform Infrared (FT-IR) Thermo-spectrophotometer. Thin layer chromatography (TLC) was done on pre-coated silica plates. Ethyl acetate and toluene mixture was used as eluent for the chromatographic purification of the compounds. Spots were visualized on exposure to UV light.
Experimental procedure for the synthesis of 3-(2-Nitrophenyl)-1-phenylprop-2-en-1-one (1a)
2-nitrobenzaldehyde (2.5g, 1 equiv) and acetophenone (2mL, 1 equiv) in acetic acid (q.s.) was stirred in a 100mL round bottom flask. Conc. sulfuric acid (4-5 drops) was added to the reaction mixture in ice cold condition and further stirred at room temperature for 24h (TLC). After the completion of the reaction, the reaction mixture was poured in ice cold water, filtered, solid was washed with water and dried in air to afford the crude product. The crude product was crystallized (MeOH) to get pure compound.
Yield: 88%, Yellow crystalline solid, mp: 157-158°C. IR spectrum (thin layer), ٧ , cm-1: 1666 (C=O), 1603 (C=C), 1511 (N=O), 1343 (N=O)cm-1. 1H NMR (400 MHz, CDCl3), δ,ppm (J, Hz): 8.12 (1H, d, J=15.90 Hz), 8.00-8.02 (3H, m), 7.69-7.74 (2H, m), 7.50-7.57 (4H, m), 7.34 (1H, d, J=15.90 Hz). 13C NMR (100 MHz, CDCl3), δ, ppm: 124.86, 126.97, 128.62, 129.13, 130.31, 131.08, 133.09, 133.56, 137.20, 140.03, 148.34, 190.22.
Elemental analysis: Found, %: %: C 71.32; H 4.15; N 5.88. C15H11NO3. Calculated, %: C 71.14; H 4.38; N 5.53. The remaining reactions were carried out following this general procedure.
Experimental procedure for the synthesis of 4,5-Dihydro-3-phenyl-5-(2-nitrophenyl) pyrazole-1-carbaldehyde (2a)
1a (3g, 1 equiv) with hydrazine hydrate (0.6ml, 1equiv) in formic acid (q.s.) was refluxed for 4 h (TLC). After the completion of the reaction, the reaction mixture was poured in ice cold water, filtered, solid was washed with water and dried in oven to afford the product.
Yield: 96%, Creamish white solid, mp: 188-189˚C. IR spectrum (thin layer), ٧ , cm-1: 1672 (C=O), 1522 (N=O), 1325 (N=O) cm-1. 1H NMR (400MHz, CDCl3), δ, ppm (J, Hz): 9.03 (1H, s), 8.15 (1H, d, J=8.00Hz), 7.75-7.77 (2H, m), 7.63 (1H, t, J=7.50Hz), 7.47-7.51 (4H, m), 7.34 (1H, d, J=7.80Hz), 6.13 (1H, dd, J=5.10 & 11.70Hz), 4.13 (1H, dd, J=11.70 & 18.30Hz), 3.22 (1H, dd, J=5.10 & 18.30Hz).13 C NMR (100MHz, CDCl3), δ, ppm: 42.89, 56.17, 125.69, 126.59, 126.76, 128.88, 128.91, 130.53, 130.98, 134.57, 135.68, 147.01, 156.35, 160.10.
Elemental analysis. Found, %: %: C 65.28; H 4.18; N 14.58. C16H13N3O3. Calculated, %: C 65.08; H 4.44; N 14.23. The remaining reactions were carried out following this general procedure.
Experimental procedure for the synthesis of 5-(2-Aminophenyl)-4,5-dihydro-3-phenylpyrazole-1-carbaldehyde(3a)
2a(2g) with excess of ferrous sulfate and ammonia in methanol (q.s.) was stirred in a 100mL round bottom flask at room temperature for 2h (TLC). After the completion of the reaction, the reaction mixture was extracted with ethyl acetate and then extract was dried to remove all ethyl acetate under vacuum. Light yellow solid obtained on cooling was washed with hexane and dried to afford 3a. The product was kept in refrigerator.
Yield: 75%, Light yellow solid, mp: 140-141˚C. IR spectrum (thin layer), ٧ , cm-1: 3354 (N-H), 3434 (N-H), 1663 (C=O), 1637 (C=N) cm-1. 1H NMR (400MHz, CDCl3), δ, ppm (J, Hz): 8.87 (1H, s), 7.77-7.80 (2H, m), 7.46-7.47 (3H, m), 7.10 (1H, t, J=7.50 Hz), 6.99 (1H, d, J=7.50 Hz), 6.71-6.78 (2H, m), 5.65 (1H, dd, J=4.20 & 11.40Hz), 4.28 (2H, NH, D2O exchangeable proton), 3.75 (1H, dd, J=11.40 & 17.70Hz), 3.42 (1H,dd, J=4.20 & 17.70Hz). 13sC NMR (100MHz, CDCl3), δ, ppm: 40.94, 54.02, 117.86, 119.88, 125.76, 126.71, 126.81, 126.85, 128.91, 128.96, 129.16, 130.75, 130.85, 144.83, 157.18, 160.36.
Elemental analysis. Found, %: %: C 72.78; H 5.48; N 15.98. C16H15N3O. Calculated, %: C 72.43; H 5.70; N 15.84. The remaining reaction was carried out following this general procedure.
Experimental procedure for the synthesis of 2-Phenylpyrazolo[1,5-c]quinazoline (4a)
3a(1g) was stirred in methanol for some time (TLC). After the completion of the reaction, the reaction mixture was dried and the crude compound obtained was purified by column chromatography using toluene as eluent to afford 4a.
Yield: 85%, Reddish solid, mp: 149-150˚C. IR spectrum (thin layer), ٧ ,cm-1: 3055 (C-H), 1618 (C=N), 1476 (C=C)cm-1. 1H NMR (400MHz, CDCl3), δ, ppm (J, Hz): 9.06 (1H, s), 7.99 (3H, d, J=7.70Hz), 7.91 (1H, d, J= 7.70Hz), 7.53-7.65 (2H, m), 7.39-7.50 (3H, m), 7.18 (1H, s). 13C NMR (100MHz, CDCl3), δ, ppm: 95.44, 119.99, 123.34, 126.73, 128.20, 128.74, 128.95, 129.34, 129.88, 132.13, 139.36, 139.72, 140.10, 155.95.
Elemental analysis. Found, %: %: C 78.56; H 4.24; N 17.42. C16H11N3. Calculated, %: C 78.35; H 4.52; N 17.13. The remaining reactions were carried out following this general procedure and the physical data for the rest of the compounds is shown below:
2-(2,5-dimethylthiophen-3-yl)pyrazolo [1,5-c] quinazoline (4b)
Yield: 80%, Yellow solid, mp: 160-161˚C. IR spectrum (thin layer), ٧ , cm-1: 2913 (C-H), 1618 (C=N), 1478 (C=C) cm-1. 1H NMR (400MHz, CDCl3), δ, ppm (J, Hz): 8.94 (1H, s), 7.88 (1H, d, J=7.64 Hz), 7.80 (1H, d, J=7.92 Hz), 7.51 (1H, m), 7.45 (1H, m), 6.98 (1H, s), 6.88 (1H, s), 2.60 (3H, s), 2.35 (3H, s). 13C NMR (100MHz, CDCl3), δ, ppm: 15.10, 15.11, 96.79, 119.88, 123.25, 126.15, 127.96, 128.66, 128.80, 129.65, 136.05, 136.10, 139.24, 139.74, 152.53.
Elemental analysis. Found, %: %: C 68.66; H 4.97; N 14.66; S 11.69. C16H13N3S. Calculated, %: C 68.79; H 4.69; N 15.04; S 11.48.
2-(5-Chlorothiophen-2-yl)pyrazolo[1,5-c]quinazoline (4c)
Yield: 80%, Yellow solid, mp: 144-145˚C. IR spectrum (thin layer), ٧ , cm-1: 1596 (C=N), 1431 (C=C), 794 (C-Cl)cm-1. 1H NMR (400 MHz, CDCl3), δ, ppm (J, Hz): 7.90-8.00 (2H, m), 7.59-7.68 (3H, m), 7.37-7.42 (2H, m), 6.80 (1H, s). 13C NMR (100MHz, CDCl3), δ, ppm: 116.6, 124.8, 126.3, 127.2, 127.5, 129.1, 130.0, 133.5, 136.7, 144.0, 147.9, 151.4.
Elemental analysis. Found, %: %: C 59.18; H 2.68; Cl 12.53; N 14.58; S 11.51. C14H8ClN3S. Calculated, %: C 58.84; H 2.82; Cl 12.41; N 14.71; S 11.22.
2-(5-Chlorohhiophen-2-yl)-7-methoxypyrazolo[1,5-c]quinazoline (4d)
Yield: 80%, Greenish white solid, mp: 148-149˚C. IR spectrum (thin layer), ٧ , cm-1: 2926 (C-H), 1600 (C=N), 1427 (C=C), 1103 (C-O), 756 (C-Cl) cm-1; 1H NMR (400MHz, CDCl3), δ, ppm (J, Hz): 8.12 (1H, s), 7.73 (1H, d, J=7.88 Hz), 7.20-7.40 (3H, m), 6.90-7.00 (2H, m), 4.00 (3H, s). 13C NMR (100MHz, CDCl3), δ, ppm: 56.31, 108.75, 117.26,119,45, 124.73, 126.59, 127.24, 128.37, 133.31, 136.83, 139.80, 144.11, 150.43, 155.18.
Elemental analysis. Found, %: %: C 56.87; H 3.36; Cl 11.01; N 13.52; S 10.34. C15H10ClN3OS. Calculated, %: C 57.05; H 3.19; Cl 11.23; N 13.31; S 10.15.
A new synthetic route for the synthesis of 2-aryl/heteroaryl pyrazolo [1,5-c] quinazolines has been developed successfully. All the synthetics were successfully characterized by using various spectroscopic techniques.
None.
The authors do not have any personal or financial interests.
None.

©2017 Sharma. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.