Journal of
eISSN: 2473-0831


Research Article Volume 4 Issue 3
Correspondence: Musaddeq Hussain, Biologics and Vaccines Research, Merck Research Laboratories, Kenilworth, New Jersey, USA, Tel +1 908-740-6434
Received: March 17, 2017 | Published: March 24, 2017
Citation: Hussain M, Bowers J (2017) A Droplet Digital PCR Method for CHO Host Residual DNA Quantification in Biologic Drugs. J Anal Pharm Res 4(3): 00107. DOI: 10.15406/japlr.2017.04.00107
The Chinese Hamster Ovary (CHO) cells are the preferred host for manufacturing therapeutic biomolecules in the biopharmaceutical industry. Host residual DNA (hrDNA) is an impurity and needs to be monitored in the purified drug to ensure purity and safety. Currently, real-time quantitative PCR (qPCR) based methods are widely employed for quantification of hrDNA, however, digital PCR technology promises higher assay sensitivity and precision. Here, we report a method where the protein drug is digested with a protease, the protease is denatured and the CHO primers and fluorescent-tagged probe from Bio-Rad Laboratories, Inc. and droplet digital PCR (ddPCR) mix are added to the reaction and nanoliter-sized droplets are generated. The droplets are then subjected to end-point PCR followed by analysis for fluorescence. Compared to qPCR, the ddPCR method shows increased sensitivity, with high precision and accuracy of determination. Additionally, the method eliminates DNA extraction step and the requirement of DNA standards in routine sample testing. The method was successfully applied to hrDNA quantification in several biologic drugs under development at Merck.
Keywords:CHO cells, monoclonal antibody, host residual DNA, biologic drugs, droplet digital PCR method
CHO, chinese hamster ovary; hrDNA, host residual DNA; ddPCR, droplet digital PCR; mAb, monoclonal antibody; PCR: polymerase chain reaction; RSD, relative standard deviation
Many therapeutic protein including monoclonal antibody (mAb) drugs are manufactured in CHO cells.1,2 The host cell derived impurities pose safety concerns and the regulatory agencies have defined acceptable levels of such impurities in the protein drug.3-6 Since the allowable limit for host residual DNA (hrDNA) is 10ng per daily dose, most manufacturers of biologic drugs often set the acceptance criteria of host residual DNA to as low as ≤1.0pg of DNA per mg of drug under development where the daily dose is unknown. As a result, very sensitive method of DNA quantification, like quantitative real-time PCR (qPCR), is employed with7 or without8,9 DNA extraction. Recently, digital PCR has become available from different vendors in different formats10,11 as an improvement over the qPCR12,13 for absolute quantification of nucleic acids. Droplet digital PCR (ddPCR) is a technology where the PCR reaction mixture is partitioned into several thousand droplets and the PCR is run to end-point, after which the numbers of positive and negative droplets of the intended target, together with Poisson’s distribution, are used in determining the target concentration without using a DNA standard curve.15
The DNA extraction efficiency impacts the sensitivity of PCR. We have developed a very sensitive CHO hrDNA ddPCR method without a DNA extraction step and instead, protease digestion is performed in a PCR plate prior to droplet generation and PCR. The primers and probe developed by Bio-Rad Laboratories, Inc., are used for the method. Compared to qPCR9 the new ddPCR method shows a lower limit of quantification (LOQ) and high precision and accuracy over the analytical range.
Materials
Seven different biologic drugs under development at Merck Research Laboratories and manufactured in CHO cells were used for this study: DS1G, DS4G, DS1I, and DS1T (about 50mg/mL) and DS4P and DS4L (about 25mg/mL) all mAb drugs, formulated in buffer containing histidine, sucrose, and polysorbate; and DSN3 a non-mAb drug, formulated at 27mg/mL in phosphate buffer containing sucrose and polysorbate. The CHO DNA standard was prepared by extracting total DNA from CHO cells using QIAamp DNA Mini kit from Qiagen (Valencia, CA). The DNA was dissolved in water and absorbance at 260nm, 280nm and 320nm was measured to determine the DNA concentration. The PCR primers and probe (part of ddPCR™ CHO Residual DNA Quantification Kit) were received from the Life Science Group of Bio-Rad Laboratories, Inc. (Hercules, CA) for pre-marketing testing. The 96-well plates for ddPCR were from Eppendorf (Hauppauge, NY) and Supermix RDQ (2X) for ddPCR and all consumables for droplet generation by Automated Droplet Generator were purchased from Bio-Rad (Hercules, CA). PCR-grade water, molecular biology grade 1X TE (10mM Tris-HCl, 1.0mM EDTA, pH 8.0) and 10X TE (100mM Tris-HCl, 10mM EDTA, pH 8.0) buffers were purchased from Sigma-Aldrich (St. Louis, MO). The KAPA Express Extract kit containing KAPA protease was purchased from KAPA Biosystems (Boston, MA). The qPCR Universal Master mix was purchased from Thermo Fisher Scientific.
CHO hrDNA ddPCR
The primers and probe for CHO hrDNA quantification was proprietary to Bio-Rad Laboratories, Inc. A 20X stock of primers and probe was provided to us for testing and was stored at –20°C in amber microtubes. For performing ddPCR, a mix was prepared so that each PCR reaction contained 12.5µL of 2X Supermix RDQ, 1.25µL of the 20X stock of primers and probe, and 1.2µL of water. The total initial volume per reaction was 25µL with 15µL of above Supermix-primer-probe mix and 10µL water for negative control samples or 5.0µL CHO DNA and 5.0µL of water for standard DNA samples. Instead of extracting hrDNA from the mAb samples, the samples were digested with KAPA protease as described in.9 Typically, the KAPA digestion was performed in a PCR plate with 2.5µL of mAb drug, 1.0µL of 10X TE buffer, 1.0µL of KAPA protease, 0.5µL of water, and either 5.0µL CHO standard DNA as spike or water for unspiked samples in a final volume of 10µL. The plate was sealed with Pierceable Foil Heat Seal using a Bio-Rad PX1 PCR Plate Sealer. The plate was then spun briefly and placed in a Bio-Rad T100 Thermal Cycler and incubated at 56°C for 60min for proteolysis, followed by 95°C for 10min for denaturation of the protease, and held at 12°C. At this point 15µL of the Supermix-primer-probe mix was added to each active well and the plate was ready for droplet generation. The 96-well PCR plate was sealed with Pierceable Foil Heat Seal, briefly spun and put in the Bio-Rad Automated Droplet Generator for making droplets according to the manufacturer’s protocol. The Automated Droplet Generator picked up 20µL out of the 25µL supplied and mixed with droplet making oil to make about 15,000 droplets for each sample and deposited in a well of a fresh PCR plate. The PCR plate with droplets was gently taken out and sealed before putting on a thermocycler for PCR. The PCR cycling conditions were: 10 min at 95°C, one cycle followed by 5 cycles each consisting of 30 sec at 95°C with 2°C/ sec ramp rate and 1 min at 53°C with 2°C/ sec ramp rate; then 40 cycles each consisting of 30sec at 95°C with 2°C/ sec ramp rate and 1 min at 70°C with 2°C/ sec ramp rate; then held at 4°C indefinitely. After PCR, the plate was transferred to the Bio-Rad QX200 Droplet Reader and fluorescence of individual droplets was read following the manufacturer’s protocol. All qPCR was performed according to the published method.9
Data analysis
Data generated by the QX200 Droplet Reader were analyzed by QuantaSoft ver. 1.7.4.0917 software by manually setting the threshold at 500 unit of fluorescence amplitude after looking at the 1D scatter of the droplets (Figure 1). Precision of DNA quantification was measured from at least three replicate PCR wells and expressed as %RSD. The accuracy was determined by measuring the DNA in spiked samples and expressed as %recovery by comparing with the results of the DNA spike alone in the same experiment.
A sensitive hrDNA method is required for monitoring host DNA impurity in biologic drugs especially with high daily dose of drug. Here, we describe development of a very sensitive ddPCR method for quantitating CHO hrDNA with primer-probe set developed by Bio-Rad Laboratories, Inc. and provided to us for testing. In order to determine whether biologic drugs could be tested in ddPCR after proteolysis similar to our previous qPCR method,9 five different mAb drug (DS1G, DS4G, DS1I, DS4P and DS4L) were spiked with CHO standard DNA and either added directly to ddPCR reaction or digested with KAPA protease before adding to ddPCR reaction without DNA extraction. A typical result of one out of four replicates of DS4P is shown in Figure 1. The fluorescent array of the droplets in Figure 1 graphically showed that,
Similar results were observed for the other four drugs (DS1G, DS4G, DS1I and DS4L). The failure with intact mAb molecules was possibly due to precipitation within the droplets during PCR thermocycling, while protease digestion eliminated the interference and drastically improved the spike recovery. The threshold, shown in Figure 1 as a red horizontal line separating the positive and negative droplets, was set manually at 500 unit of fluorescence amplitude after analyzing ddPCR results of three different experiments with three methods of threshold placement :
The manual threshold setting was found to be the most sensitive option showing minor differences from the Auto C or the ddpcRquant derived values (data not shown). All data presented here were generated with manual threshold set at 500 unit of fluorescence amplitude.
We tested the linear range and precision of the ddPCR method by serially diluting the CHO standard DNA and performing ddPCR runs on six different days. Results demonstrated a linear range from 2e4fg to 0.2fg of DNA per PCR reaction (Figure 2). The intermediate precision of the method in RSD was <30% for the whole range, and the Relative RMSE was 50%. A conversion factor for DNA copies to weight in fg of DNA was calculated by taking inverse of the slope of the standard curve, i.e., 1/ 10.15=0.1, indicating that one copy of DNA determined in the ddPCR equaled to 0.1fg of CHO DNA. The ddPCR method could now be performed without any DNA standard in the experimental run and the results of copies of DNA could simply be converted to weight of DNA using the conversion factor. Since the genome size of Chinese hamster was about 2400 Mbp or about 2400fg, the data indicated that there were about 24000 copies of the target in the genome. Compared to ddPCR, the qPCR results showed high precision over the linear range of 5e6fg to 5.0fg with %RSD of <30, and a similar Relative RMSE, when data from five different experiments were analyzed for intermediate precision. However, at the lower end of the range, from 10fg to 0.5fg, as shown in inset for Figure 2, the precision with different drugs suffered. In this range, different drug spiked with standard DNA has statistically different results (p<0.0001) from the DNA standards and each other. As a result, the LOQ for qPCR was set at 5.0fg of DNA per PCR well in our labs.
Initial assessment showed that up to 150µg of drug after KAPA protease digestion could be analyzed in ddPCR as in qPCR9 (Figure 1). In order to further assess the matrix effect on the method, an experiment was conducted with DS1G, DS4P and DS1T spiked with serially diluted DNA standards along with unspiked control followed by KAPA digestion. Results in copy number of DNA were superimposed on the standard curve in Figure 2. For mAb DS1G at 100 µg, the assay precision measured by %RSD was <20% for DNA spike to 2.0fg and 36% with 1.0fg and 58% with 0.5fg of spiked DNA; the average accuracy was 145%. For mAb DS4P at 52µg, the precision was <24% for DNA spike levels from 1e4fg to 5.0fg and was about 32% at spike level of 1.0fg; the average %recovery was 139. For mAb DS1T at 25µg, the precision was <27% for DNA spike levels from 1e4fg to 1.0fg; the average %recovery was108. The data showed that the method was reasonably precise and accurate up to 1.0fg of DNA spiked to drug and 1.0fg (which represents 10 copies of the PCR target) could be the LOQ of the method.

Figure 1 Droplet fluorescence amplitude with CHO hrDNA ddPCR method. The mAb DS4P (52µg) was either not spiked with CHO DNA (wells in the left) or spiked (right wells) and if digested with KAPA protease shown as +K in the x-axis; four replicates for each condition shown and separated by yellow vertical lines. The y-axis showed the fluorescence amplitude for each droplet in this 1D concentration plot generated by Bio-Rad QuantaSoft ver. 1.7.4.0917. The horizontal line of threshold separating positive and negative droplets was manually set at 500.
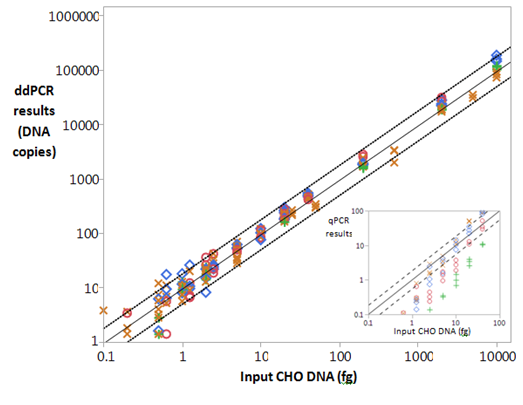
Figure 2 Linear range of CHO hrDNA ddPCR method. The linear range was determined with DNA standards only ((X), and DNA standards with KAPA-digested drugs, 100µg of DS1G (o) or 50µg DS4P (◊) or 25µg of DS1T (+). The ddPCR results are shown in the y-axis as copies of DNA detected. Based on the DNA standard only, the solid line shows the linear trendline for the mean. The dotted lines show the 99% confidence interval for the individual measurements, calculated by 2.5 RSME of a log/log fit with slope =1. The conversion factor of DNA copies to weight in fg was calculated from the inverse of the slope of the standard curve; 1/10.15 ≅ 0.1. Inset: Results for qPCR plotted against equivalent mean and confidence interval, The equivalent LOQ for qPCR, excluding DS1T and DS1G, is 5.0fg, . For all drug with ddPCR, the LOQ is 1.0fg.
Comparison of qPCR and ddPCR results showed that both methods were equally able to detect hrDNA in two out of the seven drugs tested. The hrDNA amounts in the samples, shown in Table 1, differed by 2-fold for DS4L and by about 12-fold for DSN3, however, the DNA quantity in the qPCR well for DSN3 was below the above-mentioned LOQ of 5.0fg for qPCR method. Applying the ≤1.0pg hrDNA/ mg of drug as acceptance criteria, DSN3 would fail with ddPCR method but would pass the hrDNA test with the qPCR method (Table 1). The high DNA copy number as readout and the visible positive droplets in the 1D plot (e.g., Figure 1) generated confidence in the ddPCR data. The lower LOQ of the ddPCR compared to qPCR increased the effective sensitivity of the ddPCR method by 5-fold over qPCR. Additionally, the advantage of the ddPCR method was that a standard curve was not necessary for quantification of unknown samples made routine sample testing easier. In summary, a precise and accurate ddPCR method more sensitive than the qPCR method, without DNA extraction step and requirement of DNA standards, was developed for measuring CHO hrDNA in biologic drug samples.
|
Drug |
Drug Amount Tested Per Reaction (µg) |
qPCR Results |
ddPCR Results |
||
|
Mean hrDNA (fg)/ PCR Well |
hrDNA (fg)/ mg Drug |
Mean copies of hrDNA/ PCR well |
hrDNA (fg)/ mg Drug |
||
|
DS4L |
50 |
6.21 |
123 |
32 |
63 |
|
DSN3 |
11 |
1.97* |
180 |
103 |
1200 |
Table 1 Comparison of CHO hrDNA quantification by ddPCR and qPCR. The biologic drugs were digested with KAPA protease in both ddPCR and qPCR methods and then analyzed without DNA extraction step.
Thanks to Chris Lee, Allisan Zanoni, Madhuri Ganta, and Carolyn Reifsnyder of Bio-Rad Laboratories, Inc. for providing the ddPCR™ CHO Residual DNA Quantification Kit used in this study; to Shruti Patel and Marko Stefanovic of MRL for technical help; and to Xiaoyu Yang of MRL for review of the manuscript.
The authors do not have any personal or financial interests.
None.

©2017 Hussain, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.