Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 5
Correspondence: Loipa Galán Martínez, Laboratorio de Electrofisiología, Departamento de Investigaciones, Instituto de Cardiología y Cirugía Cardiovascular, Paseo y 17, Vedado, CP 10400, La Habana, Cuba
Received: July 31, 2017 | Published: October 15, 2018
Citation: Martínez LG, González GEC. Contractile and electrical activities of dexchlorpheniramine on rat hearts. J Anal Pharm Res. 2018;7(5):575-579. DOI: 10.15406/japlr.2018.07.00285
Dexchlorpheniramine is a first-generation H1-Antihistamine, and despite numerous studies on the cardiotoxicity of other first-generation H1-Antihistamines, the effects of dexchlorpheniramine on cardiac physiology are not well known. The objective of the present study was to characterize the action of dexchlorpheniramine on the amplitude of the force of contraction, and on the electrocardiographic parameters in isolated and perfused hearts of Wistar rats. The Langendorff isolated perfused heart technique was performed and the effects of dexchlorpheniramine (3-300μmol/L, n=6) on the cardiac contraction and on the RR, QRS, QT and QTc parameters of the electrocardiogram were measured. The RR, QRS, and QT intervals increased by dexchlorpheniramine, not being so with the QTc interval. This probably suggests some type of action of dexchlorpheniramine on cardiac K+ and Na+ channels. The amplitude of the cardiac force of contraction decreased in concentration dependent manner, which suggests some effect on the Ca2+ current by dexchlorpheniramine. The IC50 estimated for the inhibition of the cardiac force of contraction was 4.12±1.64μmol/L.
Keywords: arrhythmia, cardiotoxicity, cardiovascular, dexchlorpheniramine, H1-antihistamines, QT prolongation
H1A, H1-antihistamines; hERG, human ether-a-go-go–related gene
H1-Antihistamines (H1A) act as inverse agonists that shift the balance towards the inactive state of the H1 histaminergic receptors.1 Since the 1970s reports began to appear of association between the consumption of H1A and the occurrence of cardiotoxicity reported in many revisions.2–6 Many studies have focused on elucidating which H1A and by which mechanisms they affect the functioning of the heart. Dexchlorpheniramine (D isomer of chlorpheniramine) is a first-generation AH1, is more potent than chlorpheniramine as antihistamic.7 Despite numerous studies on the cardiotoxicity of other first-generation AH1, the effects of dexchlorpheniramine on cardiac physiology are not well known. Chlorpheniramine (racemic mixture) blocks the delayed rectifier potassium channel IKr, and it lengthens the action potential, slows cardiac repolarization, and prolongs the QTc interval.3,4,8–10
Salata et al.11 founded that (+)chlorpheniramine also blocked IKr weakly, with IC50 values of 1.6μmol/L, and blocked IKs slightly (<20%) at high concentrations (10μmol/L). In chloralose-anesthetized dogs, (+)chlorpheniramine did not significantly alter the QTc interval and ventricular effective refractory period, in vivo indexes of ventricular repolarization at doses up to 3.0mg/kg IV.11
In clinic, chlorpheniramine caused a lengthened in the QT segment or Torsades de pointes tachycardia in combination with others drugs such as thioridazine,12 common cold compound medication,13 lamotrigine and citalopram,14 and with propranolol.15 Therefore, the aim of the present study was to investigate the action of dexchorpheniramine on electrical and contractile activities of isolated rat hearts.
Animals
Male adult (7-8 weeks) Wistar rats were obtained from the National Center for Laboratory Animal Reproduction (CENPALAB; La Habana). Prior to the experiment, animals were adapted for seven days to laboratory conditions (controlled temperature 25±2°C, relative humidity 60±10%, and 12h light/dark cycles). Tap water and standard diet for rodents supplied by CENPALAB were freely provided. All procedures were also conducted according to the European Commission guide-lines for the use and care of laboratory animals and approved by the Ethical Committee for Research of the Center (No. 02-2015, folio 3, book 01, 2015). The minimum number of animals (n=6) required to obtain consistent data were employed.
Isolated hearts
As previously reported,16 under pentobarbital anesthesia rat hearts were removed and placed in cold Tyrode (see below). Rat hearts were carefully dissected, mounted on a Langendorff column and perfused at constant flow (10mL/min) with a Tyrode solution of the following composition (mmol/L): 140NaCl, 2.5KCl, MgCl2, 2CaCl2, 10 Tris-hydroxymethylaminomethane, 10 Glucose (pH=7.4, gassed with O2; T=35°C). A bipolar platinum recording electrode was placed on the ventricular epicardium to record the surface electrocardiogram. Another bipolar platinum electrode was placed near the atrioventricular ring and was connected to an electronic stimulator.
The cardiac apex was fixed to a force-displacement transducer with a surgical 6-0 silk thread to record the force of contraction. Electrocardiogram and the force of contraction values were recorded at the spontaneous heart rate and at a fixed stimulus rate (200-ms RR interval). The incidence of arrhythmia was analyzed in accordance with the Lambeth Conventions (II).17
Dexchlorpheniramine and chemicals
A gift sample of dexchlorpheniramine maleate (molecular weight=390.86) raw material was supplied by BioCubaFarma, and it was diluted in the bathing solution on the day of the experiment. All other chemicals used in this experiment are of good quality from Sigma Aldrich.
Statistical analysis
Means and standard errors of means expressed the results. Student’s t- test evaluated the statistical significance for paired samples, previously checked that the data complied with the premise of normality. Differences were considered statistically significant for p<0.05. The graphics and the statistical processing were done using the software OriginPro 8 SRO v8.0724 (MA, USA).
In the registered electrocardiogram, dexchlorpheniramine significantly prolonged the QRS (Figure 1A) and RR interval (Figure 1B) at the higher concentrations of the drug. In the QRS interval, the significant statistic (p<0. 05) differences were observed since 30μmol/L, while that in the RR interval, it occurred since 100μmol/L. The Figure 2 & Figure 3 show experimental electrocardiogram registers of dexchorpheniramine and control condition with the prolongation of QRS and RR interval, respectively.

Figure1 Effects of dexchlorpheniramine on electrical activity of isolated, Langendorff-perfused rat hearts.
Effect of dexchlorpheniramine on the QRS (A) and RR (B) interval of the electrocardiogram recorded in isolated rat hearts.
The data represent means ± SEM for n=6. *p<0.05 compared to control values.

Figure 2 Effect of dexchlorpheniramine 300μmol/L (green trace) on electrocardiographic patron in isolated, Langendorff-perfused rat heart. QRS complex was widened with respect to the control condition (black trace).

Figure 3 Effect of dexchlorpheniramine at different concentrations (100 and 300μmol/L) on electrocardiographic patterns in isolated, Langendorff-perfused rat heart. RR interval was significantly prolonged.
As QRS interval, the QT interval was slowed by dexchorpheniramine in significant manner since 30μmol/L (Figure 4A). At the other hand this drug did not significantly alter the corrected QT (QTc=QT/√RR) interval (Figure 4B).
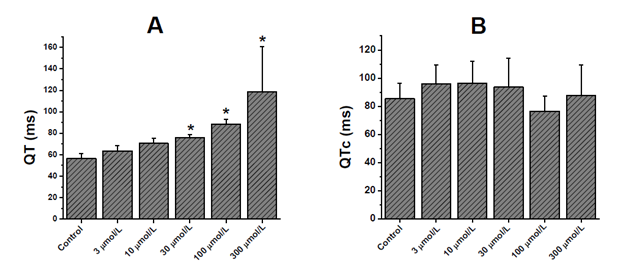
Figure 4 Effects of dexchlorpheniramine on electrical activity of isolated, Langendorff-perfused rat hearts.
Effect of dexchlorpheniramine on the QT (A) and QTc (B) interval of the electrocardiogram recorded in isolated rat hearts.
The data represent means ± SEM for n=6. *p<0.05 compared to control values.
Dexchorpheniramine induced arrhythmias in all hearts studied. It showed a tendency to bradicardic effect with increasing concentration, since RR interval was significantly prolonged (Figure 3). Moreover, dexchlorpheniramine since 10μmol/L induced ventricular premature beats (see an example in Figure 5), these were sustained or episodic in some cases.

Figure 5 Example of arrhythmic effect of dexchlorpheniramine at 10 μmol/L in isolated, Langendorff-perfused rat heart on electrocardiogram. The circle indicates the induction of a ventricular premature beat by 10μmol/L of dexchlorpheniramine. Note also the bradicardic effect.
Dexchorpheniramine showed a concentration-dependent decrease on the force of contraction of all hearts studied. At 10 μmol/L the inhibition of cardiac contraction was 52.24±11.55%. At the higher concentrations 100 y 300 μmol/L of dexchorpheniramine the percentage inhibition were 63.4±23.53% y 75.65±19.87%, respectively (Figure 6). The IC50 for this inhibition was 4.12±1.64μmol/L.
The results of this study show the cardiac action of dexchorpheniramine. This drug prolonged the QRS at higher concentrations. Many drugs that block the cardiac Na+ channel are associated with QRS prolongation.18–20 Pharmacological inhibition of the cardiac Na+ channel can be associated with intracardiac conduction delay, and may also trigger arrhythmias and can be associated with an increased mortality rate.18–20 H1A as diphenhydramine,21 and brompheniramine22 inhibited the Na+ current. It cannot discard with these results that the mechanism for which dexchorpheniramine prolongued QRS interval was the Na+ current blockade.
In the present results dexchorpheniramine significantly slowed the QT interval at the most higher concentrations. However, dexchorpheniramine did not significantly alter the QTc interval. Similar results were obtained by Salata et al.11 in chloralose-anesthetized dogs, (+)-chlorpheniramine did not significantly alter the QTc interval at doses up to 3.0mg/kg IV.11 Some H1A (clemastine, hydroxyzine, brompheniramine, chlorpheniramine, diphenhydramine, cyproheptadine, chlorcyclizine, and promethazine) lengthened the QT interval of electrocardiogram of isolated perfused feline hearts.8 In this case the concentration of chlorpheniramine for the significative lengthening of QT was 10μmol/L, less than the concentration in the present results as of 30μmol/L. For the other alkylamine, brompheniramine lengthened the QT interval since 1μmol/L.8 Katchman et al.9 considered to chlorpheniramine as low-potency hERG current blocker (IC50=13μmol/L).9 Similar results obtained Hong et al.10 where chlorpheniramine also inhibits hERG channels expressed heterologously in Xenopus oocytes with an IC50 value of ~20μmol/L.10
Drugs prolong the QT interval by blocking voltage-gated K+ channels, especially the rapid component of the delayed rectifier potassium current IKr, expressed by hERG.23 Rat hearts are believed to be less susceptible to these effects because of their dominant transient outward K+ current (Ito), which is believed to override any effect on IKr.24 However, some studies have shown that cardiotoxic drugs prolong QT interval in rodents, and electrocardiogram recording in rats has been used as a screening tool in cardiotoxicity studies.25
It needs to be noted that the translation of the results of those studies to humans has limitations. This is because rats’ hearts do not express the hERG. However, the rats’ hearts express a variant of Ether-a-go-go-Related Gene (rat ERG, also known as Kcnh2),26 which may play a role in drug induced cardiotoxicity, but further research is needed to support this notion.
In the last years, it has increased the attention on other channels that also can affect this parameter (QT interval), as Na+ channel.27 Already commented previously the report of some H1A with Na+ channles. Moreover, the therapeutic plasma concentrations of (+)-chlorpheniramine are between 12.5 and 30nmol/L,28 lower than the concentration required to produce significant contractile and electrical effects in the present results. With these limitations, it has nevertheless shown that conventional H1A can cause concentration-dependent cardiac contractile and electrical effects.
All hearts in this study experimented arrhythmias as bradycardia and ventricular premature beats since 10μmol/L of dexchlorpheniramine. Others H1A like chlorpheniramine and diphenhydramine block cardiac K+ and Na+ channles in this range of concentrations and produce arrhythmias that can become severe.5,29–30
Multicentric studies report that some H1A shown risk to cause arrhythmias including dexchorpheniramine.31,32 According to the present results, dexchorpheniramine decreased the cardiac force of contraction in concentration dependent manner, reaching a maximum of inhibition of 75.6±19.87% to the highest studied concentration (300μmol/L). Not it knows about the effects of H1A on cardiac contraction, but due to that parameter depends principally of calcium influx, some authors have shown that some H1A block cardiac calcium current. So, brompheniramine, an H1A structurally related with dexchorpheniramine, blocked the cardiac calcium current.22 With the present results it cannot affirm the effect of dexchlorpheniramine on calcium current. Future studies are needed to clarify these findings.
In this work dexchlorpheniramine shows direct contractile and electric cardiac effects in concentration dependent manner. However, these actions were observed to higher concentrations than the plasmatic concentrations of therapeutic doses. These results suggest that dexchlorpheniramine exerted a multi-ion channel–blocking action in the heart, and it that requires further studies. These conclusions may account for clinical data that have been reported previously.
This work was supported by the Cuban Ministry of Public Health (Project No. 1502301).
The authors declare no conflict of interest.

©2018 Martínez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.