Journal of
eISSN: 2473-0831


Layered double hydroxides are an emerging and intriguing class of materials having unique structural properties that allow their application in very different fields, such as catalysis, pollution control, agriculture, electronic, nanomedicine and drugs delivery. They are constituted by a sheets structure with formula M2+1-x M3+x (OH)2](An-)x/n yH2O (M2+= Zn, Mg, Fe, Ni, Co..; M3+=Al, Ga, Fe…; An- = nitrates, carbonates, chloride). The exchange of these counterions with others inorganic/organic species, producing hybrid nanocomposites, makes these systems ideal candidates for many application. The goal is to find the right cationic ratio and the proper experimental conditions (pH, temperature, time, concentrations) to make the exchange process rapid and with high yield. A large number of synthetic processes for the nanocomposites production are theoretically disposable: coprecipitation, ionic exchange, reconstruction, hydrothermal or sol-gel synthesis. We will briefly describe the structural features of LDHs and the most widespread synthetic procedures.
Keywords: Layered double hydroxides; LDH; Intercalation; Drug delivery; Catalysis
Layered double hydroxides (LDHs), also known as anionic clays or hydrotalcite-like materials, are a large family of 2D materials constituted by positively charged sheets of hydroxides intercalated by negatively charged anions. In general, the hydrotalcite structures are very robust, with a wide range of possible compositions and so with easily tunable functional properties. In fact, the most appealing aspect of these structures is the easiness to replace the anions usually located into the structure with different species that justify their application in very different fields. In fact, LDHs are used as additives in polymers, in agriculture, for pollution removal, for adsorption of organic wastes or heavy metal ions, in catalysis, photochemistry, electrochemistry and in pharmaceutics as drug delivery [1-4]. Hydrotalcite can be found as natural mineral, but many kinds of low cost and green syntheses are available.
The structure of LDH can be represented by the general formula [M1-x2+Mx3+(OH)2]x+[Ax/n]n- mH2O where the cations can be M2+= Mg, Fe, Co, Cu, Zn…, M3+=Al, Mn, Fe, Ga and An- are mainly inorganic or organic anions such as CO32-, OH-, Cl-, NO3- and others [1-4] (Figure 1). Their role is to compensate the positive charges of lamellae and guarantee the electro-neutrality of the entire network; water molecules can be also present. These structures derive from that of the mineral brucite, Mg(OH)2, in which Mg ions are located in octahedral sites with six OH- groups at the vertices, pointing the H atoms towards the interlayer spacing. The octahedra are linked by edges forming planar and neutral extended layers connected by H bonds. When the bivalent cations are partially substituted by trivalent ones, positively charged sheets form: to restore the electro-neutrality anionic species between the layers are mandatory. In these cases together with hydrogen bonds also electrostatic interactions take place, that regulates the resulting arrangement of the layers. The crystal structure deriving from the stacking of layers can be of rhombohedric or hexagonal symmetry: however most of the synthetic LDHs display rombohedral R-3 unit cell. An important request for the LDH stability is that the cations located in the octahedral sites should have similar ionic radii and in general the ratio M2+/M3+ is between 1 and 6. In principle, there is no limitations to the anionic species that can balance the positive residual charges. It has been observed that simple inorganic species with high charge/radius ratio enter more easily into the LDH layers, because they also improve the electrostatic interactions. For organic anions other factors such as their geometry, size and their mutual interaction are particularly important for the intercalation.
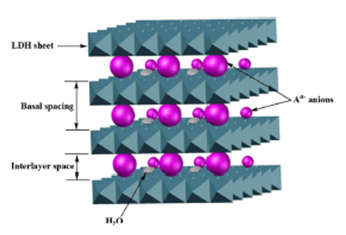
Figure 1: LDH structure, in which the hydroxyl sheets with the octahedra, the anions intercalation and the basal and interlayer spacings are evidenced.
The main synthesis methods of LDHs
In Figure 2 the most simple and widespread methods of LDHs production are shown. In the following, we briefly describe them, evidencing their strengths and weaknesses.
Co-precipitation method
This is the most employed method to synthesize LDHs, because it can be performed in a single step with high yield and starting from cheap reagents [1]. The bivalent and trivalent cations salt solutions are mixed together and with the solution of the anion to be intercalated, under continuous stirring. The pH of the mixture is maintained to the optimal basic value (pH range 6-11) by adding a solution of soda, to favor the simultaneous precipitation of cation hydroxides. To promote the intercalation of the desired anions, the preferred counter ions of cation salts are the nitrates or chlorides which possess low affinity towards the brucite layers. Important precaution to be taken are the use of de-carbonated water (to avoid the presence of CO32- anions, highly stable in the interlayer spacing) and an inert flux such as nitrogen to avoid the contact of the solution with atmosphere. Impurity phase’s formation is limited with the right choice of pH value, as well as the cations ratio (ideally between 2 and 4). A final step of reaction at moderate temperature (60-80°C) for some hours can help to increase the crystallinity of the obtained nanohybrids. Many examples of the use of this method can be found for the synthesis of nanocomposites for drug delivery, in particular for the intercalation of anti-inflammatory drugs, anti cancer agents [5], pesticides [6], amino acids [7], peptides, antibiotics [8] and others [9].
Ionic exchange
The ion-exchange method is useful when the coprecipitation method is not applicable, in particular when the chosen metal ions are unstable at high pH or when there is a strong possibility of interaction between the guest species and the metal ion [1,2]. In the anion-exchange method, the pre-formed LDH structure, usually containing Cl− or NO3- as interlayer anions, is added to a concentrated solution of the anions of interest. The obtained solution is maintained under stirring for many hours at room temperature or at about 50-70°C. The exchange efficiency varies depending on the ability of the exchanged anions to stabilize the lamellae and/or to their proportion with respect to the LDH precursor anion. It is less immediate than co-precipitation because two steps are necessary, but many examples can be found in drugs [10,11] and pesticide [12] intercalations.
Hydrothermal synthesis
The hydrothermal synthesis starts from suspension of oxides or hydroxides of M2+ and M3+ cations. A solution with the chosen acid or salt is inserted into this suspension, then the obtained dispersion is treated at high pressure and temperature in a hydrothermal reactor. The great advantage of this method, when compared with the coprecipitation one, is to avoid undesirable waste discard, which may be harmful for the environment. In addition, since no other competing anions apart hydroxides, which have very low affinity towards LDH interlayers, are present, the hydrothermal method has been found effective in pushing organic guest species into the LDH interlayers. It is also a useful method to control the final particle sizes and morphologies. In many cases, it is also used to improve the crystallinity of LDHs for a better tuning of some properties [13].
Sol-gel synthesis
In this method, the formation of a sol by hydrolysis and partial condensation of a metallic precursor is then followed by the gel formation. Metallic alkoxides, acetates, or acetylacetonates, as well as many inorganic salts can be used as metallic precursors. The properties of the obtained solid LDH depend on the hydrolysis and condensation rates of the metallic precursors and can be tuned by controlling different reaction parameters such as pH, nature and concentration of the metallic precursors, solvent and the temperature of synthesis. The material prepared by this method has well controlled pore sizes, high specific surface area and high purity [14]. However, currently, it is the less exploited method.
Reconstruction
This interesting method takes advantage of the memory effect of some LDHs. In fact, these materials, when heated at temperatures of about 400-500°C in inert atmosphere, form a mixture of metal oxides that is able to regenerate the hydroxidic layers when exposed to water [1,4]. The procedure contemplate that a LDH-CO3 sample (simple to produce) is thermally decomposed and the resulting mixture of oxides is dispersed into the desired anion solution under an inert atmosphere and by using de-carbonated water to avoid the CO32- contamination. This is the method of choice when large anions should be intercalated into LDH or when the chosen anions are not favored for intercalation by ion exchange method. It is also the preferred method for active principle intercalation, because the presence of anions, such as Cl- or NO3- , with possible negative effects into living bodies, is avoided. The incorporation of competing anions is also limited, even if particular care should be paid to the pH value that when raises too much can favor the OH- insertion. For this method, a variety of examples can be found in the literature from pesticides to vitamins and antibiotics [3].
Layered double hydroxides are 2D materials with a great potential. Currently they find application in many different fields from catalysis, agriculture, electrochemistry and nanomedicine. A proper tuning of their structural and morphological characteristics can foresee for the future even wider employment.
None.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.