Journal of
eISSN: 2473-0831


The study was focused toward synthesis, characterization and quantification of 3-Ethyl-indole impurity in Zolmitriptan formulations by Reverse Phase High Performance Liquid Chromatography method. The synthesis of a process related impurity of Zolmitriptan was successfully carried out by Fischer indole procedure. The impurity was purified by column chromatography. Characterization was done by I.R,1H-NMR,13C-NMR and GC-MS. Based on the spectral data, the structure of impurity was characterized as 3-Ethyl-indole. An efficient isocratic RP-HPLC was developed and validated according to ICH guidelines with respect to specificity, accuracy, linearity and precision. The validated HPLC method was used for detection and quantitation of 3-Ethyl-indole, a process related impurity of Zolmitriptan, from Zolmitriptan tablet formulations. The above method was found to be specific, accurate, precise, rugged and robust and can be used for routine analysis.
Keywords: zolmitriptan, fischer indole, column chromatography, iisocratic, rugged, robust, specificity, accuracy, linearity, precision
NMR, nuclear magnetic resonance; HPLC, high performance liquid chromatography; RP-HPLC, reverse phase high performance liquid chromatography; HPTLC, high performance thin liquid chromatography; TLC, thin liquid chromatography; API’s, active pharmaceutical ingredient’s; LOD, limit of detection; LOQ, limit of quantitation; FTIR, fourier transform infra red spectroscopy
In Pharmaceutical World, an impurity is considered as any other organic materials, besides the drug substances, or ingredients, arises out of synthesis or unwanted chemicals that remains with Active Pharmaceutical Ingredient’s (API’s). The impurity may be developed either during formulation or upon aging of both API’s and formulations. Presence of impurities in trace quantity in drug substance or drug product is inevitable. Therefore, their level should be controlled and monitored. They reinforce or diminish the pharmacological efficacy of the Active Pharmaceutical Ingredient’s.1
ICH defines impurities profile of a drug materials is “A description of the identified and unidentified impurities, present in a new drug substance.” For Pharmaceutical products, impurities are defined as “substance in the product that are not the API itself or the excipient used to manufacture it ” i.e. impurities are unwanted chemical that remains within the formulation or API in small amounts which can influence Quality, Safety and Efficacy, thereby causing serious health hazards.2
Qualification of the impurities is the process of acquiring and evaluating data that establishes biological safety of an individual impurity; thus, revealing the need and scope of impurity profiling of drugs in pharmaceutical research. Identification of impurities is done by a variety of Chromatographic and Spectroscopic techniques, either alone or in combination with other techniques.3‒5 There are different methods for detecting and characterizing impurities with TLC, HPTLC, and HPLC etc. Conventional Liquid Chromatography, particularly, HPLC has been exploited widely in field of impurity profiling; the wide range of detectors, and stationary phases along with its sensitivity and cost effective separation have attributed to its varied applications. Various regulatory authorities like ICH, USFDA, Canadian Drug and Health Agency are emphasizing on the purity requirements and the identification of impurities in Active Pharmaceutical Ingredient’s (API’s).6‒8 According to ICH guidelines on impurities in new drug products, identification of impurities below the 0.1% level is not considered to be necessary, unless potential impurities are expected to be unusually potent or toxic. According to ICH, the maximum daily dose qualification threshold is considered as follows; ≤ 2g/day 0.1% or 1 mg per day intake (whichever is lower) ≥ 2g/day 0.05%.9‒12
Materials reagents and chemicals
Butanaldehyde, silica gel, hydrochloric acid etc. were purchased from Merck Chemicals Pvt. Ltd. Nasik, MS, India are of AR grade. Methanol, benzene of AR grade and the acetonitrile, methanol and water of HPLC grade were purchased from Merck Chemicals Pvt. Ltd. Nasik, MS, India. The Zolmitriptan tablet formulations of different batches were purchased from local market of Kopargaon.
Melting points were determined by open capillary method and are uncorrected. The NMR spectra were recorded on sophisticated multinuclear FT-NMR Spectrometer model Advance-II (Bruker) using dimethylsulfoxide-d6 as solvent and tetramethylsilane as internal standard for1H and13C NMR. IR spectra were recorded on Shimadzu FTIR-8400S spectrophotometer using KBr disc method.
Chromatographic conditions
The quantitation of indole from formulation was carried out by HPLC method. The LC20AD Prominence Liquid Chromatography SPD20-A Shimadzu, Japan with UV-Vis detector and C18 column with dimension on 25 x 0.6cm was used for the method development with flow rate 1.0ml/min at wavelength 236nm. The methanol: acetonitrile: water in proportion of (35v:38v:27v) as a mobile phase, for development of chromatogram. The method was validation for synthesized compound and various parameters according to ICH guidelines (Q2B) were studied.
General method for 3-ethyl-indole synthesis
Synthesis was started by Fischer indole synthesis using phenyl hydrazine with appropriate aldehyde and hydrochloric acid was refluxed in presence of methanol for 2hrs and then filtered to offer indole derivative.13
Preparation of standard solution
100ppm (100µg/ml) of standard solution of synthesized compound was prepared by dissolving in methanol. 10mg of standard synthesized compound was dissolved in methanol up to 100ml. From this stock, 1ml of solution was pipette out and diluted by the solvent up to 10ml to prepare 10ppm (10µg/ml) of solution
Preparation of test solution
Test solution of formulation with different concentration from 1ppm to 10ppm was prepared using same solvent. Validation parameters were carried out for impurity by calculating range, linearity, accuracy, precision, ruggedness, robustness, LOD and LOQ as per ICH guidelines.
Analytical method validation
A suitable analytical method was developed and validated for identification. New drug development requires meaningful and reliable analytical data to be produced at various stages of development.
Preparation of mobile phase
The selection of mobile phase was according to polarity and non-polarity of solvents. The methanol: acetonitrile: water was selected as mobile phase in ratio of 35:38:27 and was filtered on membrane filter (0.45μ) to remove degassing and were stirred for 10-15min.
Preparation of stock solution standard
The stock solution was prepared according to the standard procedure viz., 10mg of synthesized compound was accurately weighed on analytical balance and using mobile phase it was dissolved to make volume up to 100 ml stock solution. The sample was prepared in the ppm in the range of 1-6ppm in concentrations respectively for the method validation by HPLC.
Preparation of sample solution (formulation)
Stock solutions of 2 different batches of Zolmitriptan marketed formulation of 100ppm in 100ml volumetric flask were prepared. For the tablet formulation 20tablets from each 2tablet batch were crushed respectively. The powder of this formulation equivalent to 10mg of the drug was used to prepare the stock solution. Further dilute to 1ppm, 2ppm, and so on, were prepared by taking 0.1ml, 0.2ml and so on of standard test solution and diluting it to 10ml. Validation experiment was performed to demonstrate system suitability, linearity, precision, accuracy study, ruggedness and robustness as per ICH guidelines.
Physicochemical properties
Thin layer chromatography
The sample preparation was done by dissolving solute in Methanol.
The Mobile phase Benzene: Methanol (6:1 v/v) Rf value- 0.80 (Figure 1).
Characterization of synthesized compound
The synthesized compound was characterized by using Advance Analytical Techniques such as UV
spectra, I.R spectra,1H-NMR,13C-NMR and GC-MS. Following are the recorded spectra.
UV- Spectra
The λmax of 3-ethyl-indole in methanol as solvent was found at 236nm. Since the compound substitute benzene chromophore, λmax confirms the structure (Figure 2).
Spectral data: The major functional group are primary amine, nitro, and carbonyl group obtained peak in IR spectra are as follows.
IR (KBr) cm-1
3341 (NH- stretching), 2850 (aliphatic –CH stretching), 2921, 2853 (aromatic C-H stretching), 1368 (C-H bending), 1297 (CH3 bending), 744,750,766 (benzene ring bending). The spectral data confirm the structure of compound.
NMR- Spectra
1H NMR: For recording the1H NMR spectra of 3-ethyl-indole, the synthesized compound was dissolved in Deuteriated Dimethyl Sulphoxide and the spectrum was recorded (Table 2).
|
S. No |
Characteristics |
Impurity |
|
|
4 |
Molecular formula |
C10H11N |
|
|
5 |
Molecular weight |
145 |
|
|
6 |
Melting point |
138°C |
|
|
7 |
Elemental Analysis |
C |
82.72% |
|
H |
7.64% |
||
|
|
|
N |
9.65% |
Table 1 Physicochemical properties of synthesized compound
|
S. No |
Group of Protons |
Chemical shift δ (ppm) |
|
a. |
Proton attached to CH2 |
2.27 |
|
b. |
Proton attached to CH3 |
3.3 |
|
c. |
Alkene proton of benzene |
6.4-6.8 |
|
d. |
Proton of NH |
5 |
|
e. |
Proton of –CH of pyrrole |
6.2 |
Table 2 1H- NMR Spectra data
The PMR data supports the structural confirmation of synthesized compound 3-ethyl-indole
GC-MS
The Q-TOF Micro mass (YA-105) spectrometer capable of recording High Resolution Spectrum (HRMS) both in Atomic Pressure Chemical Ionization (APCI) and Electron Spray Ionization (ESR) were used for quantitation of synthesized impurities. The m/e ratio was calculated for synthesized compound. m/e for the synthesized compound was found at 145 confirms the mass of the synthesized impurity.
UV method validation of impurity
UV Method was developed for synthesized compound was carried out as per ICH (Q2B) guidelines.
Range: The range was found to be 1-18µg/ml.
Linearity: Calibration curve data was constructed in the range of 1 to 18µg/ml. Beer’s law was observed over this concentration range. The correlation coefficient (R2) was found to be 0.995. The regression equation Y= 0.054x+ 0.009 was found to be linear (Table 3 & Figure 4).
|
S. No |
Concentration (ppm) |
Absorbance at 236 nm |
|
1 |
2 |
0.16 |
|
2 |
4 |
0.217 |
|
3 |
6 |
0.324 |
|
4 |
8 |
0.442 |
|
5 |
10 |
0.565 |
|
6 |
12 |
0.629 |
|
7 |
14 |
0.756 |
|
8 |
16 |
0.912 |
|
9 |
18 |
1.007 |
|
10 |
20 |
1.119 |
Table 3 Linearity of impurity by UV
Precision (Table 4).
|
S. No |
Concentration (ppm) |
Absorbance at 236nm |
Mean |
S.D |
% RSD |
|
1 |
6 |
0.315 |
0.319 |
0.0024 |
0.8777 |
|
2 |
6 |
0.321 |
|||
|
3 |
6 |
0.324 |
|||
|
4 |
6 |
0.319 |
|||
|
5 |
6 |
0.321 |
|||
|
6 |
6 |
0.318 |
|||
|
7 |
6 |
0.319 |
|
|
|
Table 4 Precision by UV
Intraday precision (Table 5).
|
S. No |
Conc. (ppm) |
Abs. At 236nm after 4hr. Reading |
Mean |
SD |
%RSD |
|
1 |
6 |
0.309 |
0.316 |
0.0035 |
1.1071 |
|
2 |
6 |
0.318 |
|||
|
3 |
6 |
0.32 |
|||
|
4 |
6 |
0.318 |
|||
|
5 |
6 |
0.317 |
|||
|
6 |
6 |
0.315 |
|||
|
7 |
6 |
0.316 |
|
|
|
Table 5 Intraday precision after 4hour
Interday precision: (Table 6).
|
S. No |
Conc. (ppm) |
Abs. At 236 nm after 24hr. Reading |
Mean |
S.D |
%RSD |
|
1 |
6 |
0.308 |
0.314 |
0.0032 |
1.1012 |
|
2 |
6 |
0.317 |
|||
|
3 |
6 |
0.318 |
|||
|
4 |
6 |
0.316 |
|||
|
5 |
6 |
0.315 |
|||
|
6 |
6 |
0.314 |
|||
|
7 |
6 |
0.314 |
|
|
|
Table 6 Interday precision after 24hours
In the result of intraday and interday precision expressed by %RSD. Since variation in %RSD was not much, state the preciseness of the method
Robustness (Table 7).
|
S. No |
Conc. (ppm) |
Abs. at 236nm (Single I) |
Abs. at 236 nm (Double II) |
Mean |
|
S.D |
|
%RSD |
|
|
|
|
|
|
I |
II |
I |
II |
I |
II |
|
1 |
6 |
0.313 |
0.315 |
0.316 |
0.319 |
0.0031 |
0.0028 |
0.981 |
0.8771 |
|
2 |
6 |
0.317 |
0.321 |
||||||
|
3 |
6 |
0.323 |
0.324 |
||||||
|
4 |
6 |
0.316 |
0.319 |
||||||
|
5 |
6 |
0.318 |
0.321 |
||||||
|
6 |
6 |
0.315 |
0.318 |
||||||
|
7 |
6 |
0.315 |
0.319 |
|
|
|
|
|
|
Table 7 Results of robustness study by change in Instrument
Robustness of the method was determined by using different instrument and the respective absorbance was noted and the result was indicated by %RSD. The method was found to be robust
|
S. No |
Conc. (ppm) |
Absorbance |
Mean |
|
S.D |
|
%RSD |
|
|
|
|
|
Analyst I |
Analyst II |
I |
II |
I |
II |
I |
II |
|
1 |
6 |
0.315 |
0.312 |
0.319 |
0.317 |
0.0028 |
0.0026 |
0.8777 |
0.8201 |
|
2 |
6 |
0.321 |
0.318 |
||||||
|
3 |
6 |
0.324 |
0.32 |
||||||
|
4 |
6 |
0.319 |
0.318 |
||||||
|
5 |
6 |
0.321 |
0.317 |
||||||
|
6 |
6 |
0.318 |
0.32 |
||||||
|
7 |
6 |
0.319 |
0.317 |
|
|
|
|
|
|
Table 8 Result of ruggedness study by change in Analyst I and II
The Ruggedness was carried out by change in analyst and the difference of %RSD is negligible indicates that the method is rugged
|
S. No |
Parameter |
SD |
%RSD |
|
1 |
Precision |
0.0028 |
0.8777 |
|
2 |
Intraday precision |
0.0035 |
1.1071 |
|
3 |
Interday precision |
0.0032 |
1.1012 |
|
4 |
Robustness |
0.0029 |
0.9293 |
|
5 |
Ruggedness |
0.0027 |
0.8489 |
Table 9 Summary of Precision
The summary of the precision is given in the above table and % RSD was found to be ≤ 2.
Accuracy: The accuracy with
known concentration of synthesized compound in formulation was determined. The recovery assessment was performed by the analysis of Zolmitriptan formulation spiked with known amount of impurity at three concentration level in triplicate of 50%. 100%, 150% and was found to be accurate as shown in above Table 10 & 11.
|
S. No |
Drug/ Formulation |
Percentage Recovery |
Mean |
S.D. |
%RSD |
||
|
|
|
50% |
100% |
150% |
|
|
|
|
1 |
Tablet I |
97.61 |
98.44 |
98.67 |
98.24 |
0.5575 |
0.5674 |
|
2 |
Tablet II |
98.19 |
98.85 |
98.39 |
98.47 |
0.3384 |
0.3439 |
Table 10 Result of recovery study by UV
|
S. No. |
Drug / Formulation |
Amount of Drug (µg/ml) |
Amount of Impurity Added (µg/ml) |
Amount Recovered(µg/ml) |
|
1 |
Tablet I |
10 |
5 |
14.63 |
|
10 |
10 |
19.68 |
||
|
10 |
15 |
24.66 |
||
|
2 |
Tablet 2 |
10 |
5 |
14.72 |
|
10 |
10 |
19.77 |
||
|
|
|
10 |
15 |
24.54 |
Table 11 Recovered amount of impurity by UV
Limit of detection:
Limit of quantitation:
The LOD and LOQ were found to be 172.2ng and 522.0ng respectively, which indicate the sensitivity of method (Table 12).
|
S. No |
Parameter |
Observation |
|
1 |
Linearity range |
1-18 μg/ml |
|
2 |
Slope |
0.0549 |
|
3 |
Intercept |
0.0095 |
|
4 |
Correlation coefficient |
0.995 |
|
5 |
LOD |
0.17227 |
|
6 |
LOQ |
0.52203 |
Table 12 Summary of method validation
The developed UV method was validated as per ICH guidelines and the method is simple, cost effective to determine 3-ethyl-indole impurity in tablet formulation of Zolmitriptan for routine analysis
HPLC method validation
The ICH Q2B guidelines discuss the analytical method validation on HPLC. Currently the vast majority of process-related impurity determinations are performed by HPLC. It offered the desired sensitivity for trace level determinations with a high degree of automation. A wide variety of stationary phases and operation modes make HPLC applicable to all drug classes. The typical detection limits for process-related impurities by HPLC are 0.1% or lower and this can be routinely met in the majority of circumstances using conventional UV detectors. These methods involved the prediction of likely impurities within the synthetic process, their isolation and identification by suitable analytical techniques. The last step of the present study was to develop, validated HPLC method for detection and quantification of 3-ethyl-indole impurity in tablet formulations.
HPLC chromatograph of zolmitriptan (Figure 5 & Table 13).
|
|
Reten. Time [min] |
Area [mV.s] |
Height [mV] |
Area [%] |
Height [%] |
WO5 [min] |
|
1 |
1.46 |
2.424 |
0.289 |
0.1 |
0.3 |
0.13 |
|
2 |
1.85 |
11.705 |
1.278 |
0.7 |
1.1 |
0.11 |
|
3 |
4.063 |
37.267 |
3.919 |
2.2 |
3.4 |
0.15 |
|
4 |
4.627 |
25.732 |
2.608 |
1.5 |
2.3 |
0.17 |
|
5 |
4.817 |
52.839 |
5.63 |
3.1 |
4.9 |
0.14 |
|
6 |
9.587 |
1568.718 |
100.231 |
92.3 |
88 |
0.23 |
|
|
Total |
1698.684 |
113.955 |
100 |
100 |
|
Table 13 The Retention time of Zolmitriptan was 9.5min
HPLC chromatogram of synthesized compound (Figure 6& Table 14).
|
|
Reten. Time [min] |
Area [mV.s] |
Height [mV] |
Area [%] |
Height [%] |
WO5 [min] |
|
1 |
1.863 |
9.288 |
0.859 |
5.6 |
8 |
0.15 |
|
2 |
4.65 |
13.445 |
0.993 |
8.1 |
9.3 |
0.21 |
|
3 |
8.223 |
8.412 |
0.653 |
5.1 |
6.1 |
0.19 |
|
4 |
10.597 |
135.253 |
8.174 |
81.3 |
76.5 |
0.25 |
|
|
Total |
166.397 |
10.678 |
100 |
100 |
|
Table 14 HPLC chromatogram of synthesized compound
The retention time of impurity was 10.5min and it shows a single peak which indicates purity of compound.
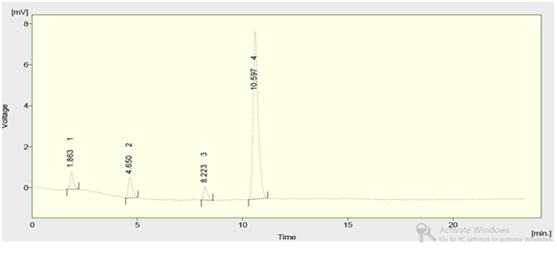
Figure 6 HPLC chromatogram of synthesized compound.
The retention time of impurity was 10.5 min and it shows a single peak which indicates purity of compound.
HPLC chromatogram of tablet (Figure 7 & Table 15).
|
|
Reten. Time [min] |
Area [mV.s] |
Height [mV] |
Area [%] |
Height [%] |
WO5 [min] |
|
1 |
1.87 |
19.233 |
2.024 |
10.7 |
16.1 |
0.12 |
|
2 |
4.643 |
39.227 |
2.71 |
21.9 |
21.6 |
0.22 |
|
3 |
9.687 |
120.869 |
7.84 |
67.4 |
62.4 |
0.23 |
|
|
Total |
179.33 |
12.573 |
100 |
100 |
|
Table 15 HPLC Chromatogram of Zolmitriptan Tablet
The retention time of Zolmitriptan tablet was found at 9.6min.
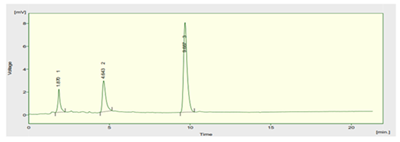
Figure 7 HPLC Chromatogram of Zolmitriptan Tablet.
The retention time of Zolmitriptan tablet was found at 9.6min.
Optimized chromatographic condition (Table 16).
|
Chromatographic Conditions |
Shimadzu HPLC System |
|||
|
Mobile phase |
Methanol: Acetonitrile: Water (35:38:27) |
|||
|
Column |
ARP-C18 (250 mm X 4.6 mm), 5μ column |
|||
|
Flow rate |
1ml/min |
|||
|
Wavelength detection |
236nm |
|||
|
Injection volume |
20μl |
|||
|
Temperature |
Ambient |
|||
|
Retention time |
10.5min |
|||
|
Run time |
15min |
|||
Table 16 Optimized chromatographic condition for RP-HPLC
Linearity (Figure 8 & Table 17).
|
S. No |
Concentration (ppm) |
Area (mill volts) at 236nm |
|
1 |
1 |
123.17 |
|
2 |
2 |
215.68 |
|
3 |
3 |
312.27 |
|
4 |
4 |
419.19 |
|
5 |
5 |
513.62 |
|
6 |
6 |
618.54 |
Table 17 Result of Linearity by HPLC (Peak area vs. Conc.)
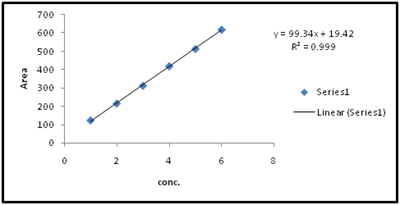
Figure 8 Graph of linearity of synthesized compound by HPLC.
The linearity of the proposed method was estimated by regression analysis at six concentration levels in the range of 1-6µg/ml for intermediate. The correlation coefficient (R2) was found to be 0.999 and intercept Y= 99.34x+19.42 was linear.
Precision: The precision of the intermediate was quantified for repeated concentration of 4µg/ml in range and was reliable with their area of chromatogram as shown in above table. The Standard deviation (SD) and Relative standard deviation (RSD) was found to be 1.331 and 0.317 respectively (Table 18).
|
S. No |
Concentration (ppm) |
Peak area (mV) at 236nm |
Mean |
SD |
%RSD |
|
1 |
4 |
419.19 |
419.89 |
1.331 |
0.317 |
|
2 |
4 |
418.98 |
|||
|
3 |
4 |
421.9 |
|||
|
4 |
4 |
419.9 |
|||
|
5 |
4 |
418.39 |
|||
|
6 |
4 |
421.01 |
|
|
|
Table 18 Precision by HPLC
Intraday precision after 4hours (Table 19).
|
S. No |
Conc. (ppm) |
Peak Area after 4hour at 236nm |
Mean |
S.D |
%RSD |
|
1 |
4 |
418.83 |
417 |
2.25 |
0.539 |
|
2 |
4 |
413.15 |
|||
|
3 |
4 |
418.39 |
|||
|
4 |
4 |
417.12 |
|||
|
5 |
4 |
415.86 |
|||
|
6 |
4 |
418.99 |
|
|
|
Table 19 Result of Intraday precision after 4 hours
Interday precision after 24hours (Table 20).
|
S. No |
Conc. (ppm) |
Peak Area after 24hour at 236nm |
Mean |
S.D |
%RSD |
|
1 |
4 |
423.12 |
424.42 |
1.76 |
0.414 |
|
2 |
4 |
422.6 |
|||
|
3 |
4 |
426.66 |
|||
|
4 |
4 |
424.24 |
|||
|
5 |
4 |
423.42 |
|||
|
6 |
4 |
426.53 |
|
|
|
Table 20 Intraday precision after 24hours
The intra and interday precision was carrying out and difference in %RSD was found not much varies and remains less than 2% indicate preciseness of method.
Robustness (Table 21).
|
S. No |
Conc. (ppm) |
Peak Area (mV) 0.8ml/min |
Mean |
S.D |
%RSD |
|
1 |
4 |
787.12 |
789.07 |
3.122 |
0.395 |
|
2 |
4 |
784.96 |
|||
|
3 |
4 |
793.21 |
|||
|
4 |
4 |
786.68 |
|||
|
5 |
4 |
791.48 |
|||
|
6 |
4 |
789.07 |
|
|
|
Table 21 Results of Robustness study by change in flow rate
At flow rate of 0.8 ml/min
The robustness of the Intermediate was performed for change in flow rate upto 0.8 ml/min and method was robust with standard deviation 3.122 and relative standard deviation 0.395.
|
S. No |
Conc. (ppm) |
Peak Area (mV) 0.8ml/min |
Mean |
|
S.D |
|
%RSD |
|
|
|
1 |
4 |
787.12 |
789.07 |
3.122 |
0.395 |
||||
|
2 |
4 |
784.96 |
|||||||
|
3 |
4 |
793.21 |
|||||||
|
4 |
4 |
786.68 |
|||||||
|
5 |
4 |
791.48 |
|||||||
|
6 |
4 |
789.07 |
|||||||
|
S. No |
Conc. (ppm) |
Peak Area in mV |
Mean |
|
S.D |
|
%RSD |
|
|
|
|
|
Analyst I |
Analyst II |
I |
II |
I |
II |
I |
II |
|
1 |
4 |
419.19 |
418.8 |
419.94 |
420.66 |
1.392 |
1.481 |
0.3314 |
0.352 |
|
2 |
4 |
418.98 |
420.18 |
||||||
|
3 |
4 |
421.9 |
420.36 |
||||||
|
4 |
4 |
419.86 |
421.79 |
||||||
|
5 |
4 |
418.39 |
419.93 |
||||||
|
6 |
4 |
421.34 |
422.98 |
|
|
|
|
|
|
Table 22 Results of Ruggedness study by change in analyst
The ruggedness of the Intermediate was carried out for change in analyst and method was found to be robust.
|
S. No |
Parameter |
SD |
%RSD |
|
1 |
Precision |
1.331 |
0.317 |
|
2 |
Intraday precision |
2.25 |
0.539 |
|
3 |
Interday precision |
1.76 |
0.414 |
|
4 |
Robustness |
3.122 |
0.395 |
|
5 |
Ruggedness |
1.436 |
0.341 |
Table 23 Summary of Precision
The summary of the precision is given in the above table and % RSD was found to be ≤ 2.
Accuracy
Accuracy study was performed by the recovery method. The results demonstrate that the percentage recovery in tablet due to the presence of impurity in the tablet. Percentage recovery was found to be more at higher concentration level a compare to lower concentration level (Table 24).
|
S. No |
Drug / Formulation |
Percentage Recovery |
Mean |
S.D. |
%RSD |
||
|
|
|
50% |
75% |
100% |
|
|
|
|
1 |
Tablet I |
99.22 |
101.3 |
103.79 |
101.43 |
2.288 |
2.255 |
|
2 |
Tablet II |
99.25 |
101.68 |
103.13 |
101.3 |
1.969 |
1.934 |
Table 24 Result of recovery study by HPLC
Limit of detection:
Limit of quantitation:
The LOD by HPLC was 446.2ng and that of LOQ 135.2ng the method is more sensitive and selective.
System suitability parameters
To verify that analytical system is working properly and can give accurate and precise results the system suitability parameters are to be set and it was found to be in stated range (Table 25).
|
Property |
Values |
Required Limits |
|
Retention time (tR) |
10.59 |
RSD ≤ 1% |
|
Theoretical plates (N) |
10224 |
N ≥ 2000 |
|
Resolution (R) |
6.36 |
R ≥ 2 |
Table 25 System Suitability Parameters of synthesized compound
To verify that analytical system is working properly and can give accurate and precise results the system suitability parameters are to be set and it was found to be in stated range.
Summary of method validation parameters of HPLC (Table 26).
|
S. No |
Parameter |
Observation |
|
1 |
Linearity range |
1-6 μg/ml |
|
2 |
Slope |
98.44 |
|
3 |
Intercept |
21.54 |
|
4 |
Correlation coefficient |
0.999 |
|
5 |
LOD |
0.4462 |
|
6 |
LOQ |
0.1352 |
Table 26 Summary of Method Validation Parameters of HPLC
Summary of retention time and asymmetry (Table 27).
|
S. No |
Compound |
Retention Time |
Asymmetry |
|
1 |
Zolmitriptan |
9.5 |
1.8 |
|
2 |
Impurity |
10.5 |
1.81 |
|
3 |
Zolmitriptan Tablet |
9.6 |
1.78 |
Table 27 Summary of retention time and asymmetry
Quantitation of synthesized compound
Impurity was not found in bulk and in tablet I & II it was found to be 0.28%.and 0.33% respectively. As per the ICH limit the amount of impurity is more than 0.1% indicates that the impurity found in tablet formulations is potential impurity (Table 28).
|
S. No |
Formulation |
Quantisation of Impurity |
|
1 |
Zolmitriptan tablet I |
0.28% |
|
2 |
Zolmitriptan tablet II |
0.33% |
Table 28 Quantisation of process related impurity of Zolmitriptan in tablets
None.
Author declares there are nno conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.