Journal of
eISSN: 2473-0831


A simple, selective, linear, precise, and accurate RP-HPLC method was developed and validated for the Quantitation of Fingolimod Hydrochloride from bulk and formulations. Chromatographic separation was achieved isocratically on Zorbax Plus C8 column (250×4.6mm, 5μ particle size) using a mobile phase, Acetonitrile and Di-butyl ammonium phosphate buffer in the ratio of 45:55v/v. The flow rate was 2.0ml/min and effluent was detected at 198 and 100μl of sample was injected. Linearity was observed in the concentration range of 0.224-1.68μg/ml. The percentage RSD for precision and accuracy of the method was found to be less than 2%. The method was validated according to the ICH guidelines with respect to specificity, linearity, accuracy, precision and robustness. The method developed can be used for the routine analysis of Fingolimod hydrochloride. BCS class of Fingolimod was determined by testing solubility, dissolution profile and lipophilicity by partition coefficient.
Keywords: fingolimod, sclerosis, hydrochloride, modulating, analys
RP, reverse phase; HPLC, high-performance liquid chromatography; UV, ultra violet; LC, liquid chromatography; MS, mass spectrometry; DAD, diode array detectors; LOD, limit of detection; LOQ, limit of quantification; RSD: reflex sympathetic dystrophy; DSC: differential scanning calorimetry
Fingolimod (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1, 3-diol hydrochloride) is an immune modulating drug, it is used mainly for multiple sclerosis. It reduces the rate of relapses in relapsing-remitting multiple sclerosis by over half, but has serious adverse effects (Figure 1).1–3
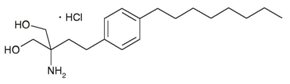
Figure 1 Structure of Fingolimod (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1, 3-diol hydrochloride).
This paper is composed of two parts. The first part focuses on Quantitative determination of Fingolimod hydrochloride using RP-HPLC-UV in the concentration of dissolution instead of LC/MS in Fingolimod HGC. The second part is a study of Fingolimod hydrochloride dissolution profile in different media compared with reference drug and the effect of excipients on dissolution.
Instrumentation, reagents and chemicals
Agilent 1260 HPLC system equipped with Zorbax Plus C8, 250x4.6mm, 5μm column, DAD detector N-dibutyl amine, orthophosphoric acid of analytical Grade, water and acetonitrile (HPLC grade) Fingolimod hydrochloride Working standard.
Preparation of buffer solution (Dibutyl ammonium phosphate)
Add 8.3ml Orthophosphoric acid to 20ml N-dibutyl amine while stirring and addition of water to 100ml then adjust to pH 2.5 then complete the volume to exactly 1000ml purified water.
Mechanism of detection
Dibutyl ammonium phosphate is ion-pair reagent which used as mobile phase to attract to Fingolimod hydrochloride molecules that result in demolishing tailing in Fingolimod peak and increase UV absorbance of whole molecule hence it increase method sensitivity up to 200mg/ml (Figure 2).
Chromatographic condition
Fingolimod was eluted in Zorbax Plus C8, 250x4.6mm, 5μm column using a mobile phase mixture of buffer and acetonitrile in the ratio of 55:45% v/v at temperature 30°C. The lambda max of the drug in mobile phase was 198nm, so column outlet was monitored at 198nm. The injection volume was 100μL, flow rate 2.0ml/min and the total runtime was 5min.
Standard solution preparation
Dissolve accurately weighed 28mg Fingolimod Hydrochloride in to a 250ml volumetric flask, add about 150ml of solvent (Acetonitrile: water 1:1) then sonicate for about 10 min then complete to volume with the same solvent, then dilute 1.0ml of Solution to 100ml with the mobile phase (Figure 3).
Construction of calibration curve
The standard Fingolimod Hydrochloride solution was further diluted in 100ml volumetric flask to obtain various concentrations ranging from 0.224 to 1.68μg/ml. From this each standard solutions 100μL was injected in to the HPLC system. The chromatograms were recorded. The concentrations of the Fingolimod Hydrochloride in μg/ml is taken in x-axis and peak area of the individual concentrations of standard solution were taken in y-axis. The calibration graph was plotted (Figure 4) (Table 1).4,5
|
Parameter |
Values μg/mL |
|
Concentration |
0.224 - 1.68 |
|
Slope |
300.09 |
|
Intercept |
8.54 |
|
R2 |
0.9992 |
Table 1 Parameters for Fingolimod Hydrochloride.
Limit of quantization and limit of detection
LOQ and LOD were calculated using HPLC Agilent Chemstation to find the ratio of signal to noise 10:1 and 3.3:1 for LOQ and LOD respectively. The limit of Quantitation is found to be 112mg/ml while the limit of detection is 56mg/ml
Precision
To demonstrate agreement among results, a series of measurements are done with Fingolimod hydrochloride ten replicate injections of the specific standard at various time intervals on the same day were injected into the chromatograph and the value of %RSD was found to 1.26%. In inter-day precision same standard was injected on different days and the found %RSD was 1.68%.4,5
Accuracy
Accuracy was done by recovery study using standard addition method, known amount of standard Fingolimod into pre-analyzed samples and subjected to proposed HPLC method. The results of recovery studies are shown in (Table 2s).4,5
|
Working Concentration Percent |
Working Concentration (mc/ml) |
% Recovery |
|
80% |
0.896 |
101.79% |
|
100% |
1.12 |
99.54% |
|
120% |
1.344 |
98.53% |
Table 2 Measurement of accuracy by standard addition method.
Dissolution profile
The formula of Fingolimod capsule contains only mannitol and magnesium stearate.6 Dissolution is performed through three standard media using apparatus 1 (Basket) and 100. RPM at 37.5°C and the volume of media is 500ml the sample directly injected to HPLC (Figure 5) (Table 3).7
|
Time (Min) |
Test Release |
Reference Release |
|
5 |
86.72 |
88.42 |
|
10 |
96.83 |
93.36 |
|
15 |
100.05 |
96.09 |
|
30 |
100.27 |
99.73 |
Table 3 Dissolution parameters Fingolimod capsule contains only mannitol and magnesium stearate.
Dissolution in pH 2.0 media the samples gives more than 85% in the first 5min. While in acetate media pH 4.5 the dissolution rate decreased but still more than 85% in 30min but it is less than 80% after 15min. the dissolution in acetate media was repeated after removal of magnesium stearate from the capsule to find out that dissolution rate exceed 85% in the first 15min (Table 4) (Figure 6).
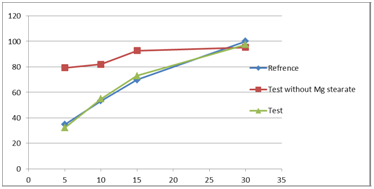
Figure 6 Dissolution profile for Fingolimod capsule in the absence of magnesium stearate with different pH (2.0&4.5) by using HPLC.
|
Time |
Test (No Magnesium Stearate) Release |
Test Release |
Reference Release |
|
5 |
79.20 |
32.25 |
97.51 |
|
10 |
81.94 |
54.95 |
53.33 |
|
15 |
92.75 |
72.96 |
69.80 |
|
30 |
95.25 |
97.51 |
100.04 |
Table 4 Dissolution parameters for Fingolimod capsule in the absence of magnesium stearate with different pH (2.0&4.5) by using HPLC.
On the other hand in phosphate media pH 6.8 the dissolution does not exceed 25% after 30min and these results are very similar to reference drug of Novartis. However it was observed that the dissolution of Fingolimod Hydrochloride doubled to reach 50% after 30 min in Phosphate media pH 6.8 unless magnesium stearate is added in the formula (Table 5) (Figure 7). Magnesium stearate in acidic media has no effect due to high solubility of Fingolimod hydrochloride in acidic media.
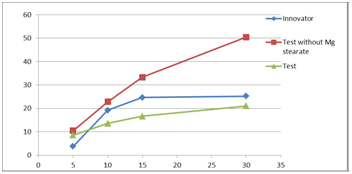
Figure 7 Dissolution profile for Fingolimod capsule in the absence of magnesium stearate with pH: 6.8 by using HPLC.
|
Time (Min) |
Test Without Mg Stearate Release |
Test Release |
Reference Release |
|
5 |
10.51 |
8.56 |
3.74 |
|
10 |
22.85 |
13.59 |
19.18 |
|
15 |
22.85 |
16.66 |
24.68 |
|
30 |
50.41 |
21.1 |
25.23 |
Table 5 Dissolution parameters for Fingolimod capsule in the absence of magnesium stearate with pH: 6.8 by using HPLC.
Influence of magnesium stearate on fingolimod hydrochloride
During dissolution it was observed that absence of magnesium stearate from the formula rises the rate of dissolution especially in acetate and phosphate media therefore IR test and Differential scanning Calorimetry (DSC) to assure the relation either hydrophobic effect or hydrogen bonding.8
IR scanning of Fingolimod and Magnesium stearate mixture show that the peak of O-H at 3400cm-1 in Fingolimod IR Spectrum has been diminished and the strength of C=O peak in magnesium stearate IR spectrum at 1650 cm-1 has decreased in mixture sample spectrum (Figure 8–11).9
This interaction has been confirmed by performing DSC for Fingolimod hydrochloride and magnesium stearate mixture which show increase in heat absorbance for both magnesium stearate and Fingolimod hydrochloride compared with each standard separately which indicates presence of hydrogen bonding. On the other hand, hydrophobic effect is always accompanied with decrease in heat absorbance (Figure 12).10,11
The developed RP-HPLC method was validated and the system suitability studies were performed and all parameters combined with the simplicity and ease of operation ensures that the validated method can successfully be used for routine analysis of Fingolimod hydrochloride in bulk and capsule dosage formulation. Although magnesium stearate has influence on Fingolimod hydrochloride solubility, it has no effect on its bioavailability.
None.
The author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.