International Journal of
eISSN: 2574-8084


Case Series Volume 8 Issue 2
1Radiotherapy Department, Instituto Nacional Oncologia y Radiobiologia, Havana, Cuba
2J.M. Marquez Hospital, Cuba
3Clinical Investigations Department, Instituto Nacional Oncologia y Radiobiologia, Cuba
4Oncopediatric Department, Instituto Nacional Nacional Oncologia y Radiobiologia, Cuba
Correspondence: Jose Alert, Instituto Nacional Oncologia y Radiobiologia, 17 number 158, apt 8, Vedado, Havana, Cuba , Tel 53-78330584
Received: April 09, 2021 | Published: June 19, 2021
Citation: Alert J, Chon I, Valdes J, et al. Very long-term of survival, 5 years and more in diffuse intrinsic pontine brainstem gliomas in children and adolescents treated with Radiotherapy and Nimotuzumab. Int J Radiol Radiat Ther. 2021;8(2):86-90. DOI: 10.15406/ijrrt.2021.08.00299
Diffuse intrinsic brainstem gliomas have a bad prognosis, and short-term survival time. Radiotherapy has been the principal treatment, and chemotherapy has not improved outcome. The anti –EGFR monoclonal antibody Nimotuzumab combined with Radiotherapy was tested in a series of 41 children and adolescents with diffuse intrinsic pontine gliomas (DIPG) included between January 2008 and December 2015 and a follow-up till January 2021.They were irradiated in the Instituto Nacional de Oncologia y Radiobiologia, Havana, Cuba with a median dose of 54 Gy. Nimotuzumab was applied at a dose of 150 mg/m2, weekly during the period of irradiation, then every 2 weeks by 8 doses, and them monthly for 1,2 or more years. A response was observed in 87.8% of patients. Prolonged use of Nimotuzumab was feasible and well tolerated. Median age at diagnosis was 7 years old, median survival was 18.8 months. There were minor toxicities, only Grade I or II. Survival rate at 5 years was 34.1%, stablished till years or more. Two relapsing patients were re-irradiated. The combination of irradiation and Nimotuzumab is an option to increase survival in DIPG.
Keywords: Irradiation of brainstem glioma, Nimotuzumab in brainstem gliomas, very long survival, re-irradiation
Brainstem tumors in children and adolescents are in most cases diffuse intrinsic pontine gliomas (DIPG), with a defined clinical presentation (multiple bilateral cranial nerve deficits, specially VI and VII, corticospinal tract deficit and ataxia) and characteristic appearance in imaging findings, so histological confirmation are often considered unnecessary.1-9 Focal radiotherapy (RT) has been the principal treatment, because surgical resection is not possible, but it has shown a low degree of efficacy, with short-term response and a median survival time of one year or less.In general, 2 and 5-year survival rates from the diagnosis are 10% and 1%, respectively.1-3,6,10,11 DIPG must be distinguished from other subsets of pontine gliomas, such as focal tumors which are described with better prognosis and longer survival.12-15 The association of radiotherapy and chemotherapy, in general, has not improved survival.6,16-21
We investigate the association of radiotherapy and Nimotuzumab, formerly called h-R3, an humanized monoclonal antibody developed at the Centro de Inmunologia Molecular, in Havana, Cuba and tested the hypothesis that this combination will improved survival. The antibody was obtained by humanization of the murine antibody EGF/R3.22 Because Nimotuzumab has a 10 fold lower affinity to EGFR as compared to Cetuximab, its capacity to bind EGFR is heavily dictated by cell receptor density.23 A distinguishing feature of Nimotuzumab compared to other MAbs of the EGFR class, is the lack of severe skin toxicity and the possibility to be used beyond progression.1,24,25 Nimotuzumab preclinical and clinical characterizations has been summarized before.26-29 Previous reports with the use of RT and Nimotuzumab show that this association is useful and even improve survival in DIPG.1,4,6,24,3-34
Long-term survival with DIPG is defined to occur 2 or 3 years since the time of the initial treatment, and Very Long-term survival is defined to an overall survival (OS) of 5 years or more.2,3,6,8,13,21,35-37
This is a prospective, non-randomized clinical study with a treatment group of 41 children and adolescents (age range between 2 to 18 years old), recruited from January 2008 to December 2015, and a follow-up till January 2021, with the diagnosis of DIPG documented clinically and by imaging (MRI,CT scan). Biopsy and histology confirmation was not a requirement of this study. All patients had a history of cranial nerve paresis, ataxia, lower or upper extremity paresis and characteristics imaging showing tumor primarily located in the pons, and infiltrating diffusely the pons. Others inclusion criteria were Karnofsky-Lansky status ≥30%, no pregnancy or breast- feeding. Patients with focal lesions of the brainstem were not elected for the study, they were also excluded those who had received prior chemotherapy or RT. Latter, we decided to include patients, no matter if Karnofsky-Lansky were 20% or less.
The study was approved by the Ethical Institution Committee at the Instituto Nacional de Oncologia y Radiobiologia (INOR) in Havana, and the Informed Consent obtained from the patients’ parents. All patients were irradiated at the INOR and received the monoclonal antibody Nimotuzumab at INOR or at Pediatric Hospital “Juan M. Marquez“, also in Havana.
No surgical treatment was feasible; in 3 patients there were pathological results by biopsy; in all patients,tumor was extended to the pons.
Patients received RT in a lineal accelerator, Gross Tumor Volume (GTV) was defined as the visible tumor, either by MRI or CT, Clinical Target Volume (CTV) accounted for subclinical microscopic disease, generally 1.5 cm from the GTV and planning Target Volume (PTV) was0.3-0.5 cm from GTV, and could vary according to Organ at Risk. RT doses ranged from 54 to 59,8 Gy, with 1,8Gy dose per fraction, 5 doses per week. Six patients were planned with IMRT and the rest with 3D CT, in all patients a thermoplastic mask was fitted. Thirty patients (73,2%) received dexamethasone during irradiation time, and radiation dose was recorded weekly.
Nimotuzumab was administered at a dose of 150 mg/m2 (IV) weekly during the time they were receiving RT, beginning in the first day of irradiation, then every 2 weeks per 8 doses, then monthly for one year. In some of the patients, the application of Nimotuzumab was prolonged for 2 o more years, depending of the Institution which made the follow-up. Categorical data were expressed as percent; survival data were estimated using the Kaplan-Meier method with long rank test. The Youden Index was applied to obtain the cut-off point signs of symptomatic improvement dose. Values with p<0.05 was considered statistically significant.
All patients completed the irradiation plan, except 2 who experienced progression of the disease and died with doses of 26 and 34,2 Gy. When there were interruptions in the time of irradiation, the dose of 54 Gy was increased to 57 Gy or more.
A total of 36 patients (87.8%), of which11 patients of the long-term follow- up and 25 of the rest (deceased), received the complete Nimotuzumab schema: interruptions of Nimotuzumab administration were because patients did not concur to the treatment during follow-up in a timely manner, but those patients received a minimum of 80% of the doses of the Nimotuzumab.
Two patients were re-irradiated for relapse of the tumor: one at 4 years of the first irradiation with a dose of 54 Gy, the other received 34,2 Gy 3 years after first irradiation; procedures were the same for the first treatment, the bean geometry for re-irradiation was chosen in order to protect previous irradiated areas.
The median age at diagnosis of the 41 patients included in the series was 7 years old ( 95% CI, 5-9) with a range between 2 and 18 years old .In all cases tumor was extended to the pons; 22 were male patients (53.7%) and 19 female patients (46.3.%).
Karnofsky-Lansky Performance Status was evaluated at inclusion: KLPS<60, n=11 (26.8%), KLPS= 60, n=14 (34.1% ), KLPS>60, n=16 ( 39.1%).
According to RT dose: the range more used was 54-57 Gy (n=17, 41.5%); 15 received 57.1-59 Gy (36.6 %) and 9 patients were irradiated with a dose higher than 59 Gy (22.9 %) (Figure 1).
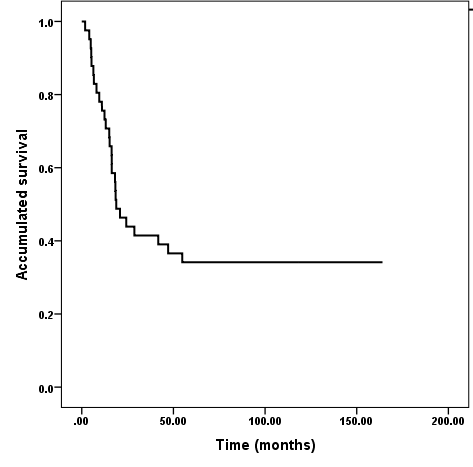
Figure 1 Overall survival. Diffuse intrinsic pontine brainstem gliomas in children and adolescents treated with Radiotherapy and Nimotuzumab.
Median dose to first signs of symptomatic improvement was 1,700 cGy, (95%CI, 1,200-2,200) in the group of 14 patients with very long OS. The median dose in the deceased patients was 1,980 cGy (95%CI, 1,110-2,550). There was no difference with statistical significance (p= 0.355). The point cut-off was established as 1,700 cGy. Although univariate analysis for range of improvement dose had not reached difference with statistical significance, it’s clinically important to note that for patients that obtained first sign of symptomatic improvement with dose less or equal to 1,700, the median of time survival was not reached. The survival rates for patients that got improvement with a range dose ≤1,700cGy, were 75.0%, 66.7% and 58.3% versus 75.0%, 36.7% and 25.0% at 1, 2 and 5 years, for patients that obtained first signs of symptomatic improvement with range of dose ≥1,700 cGy, respectively (Figure 2).
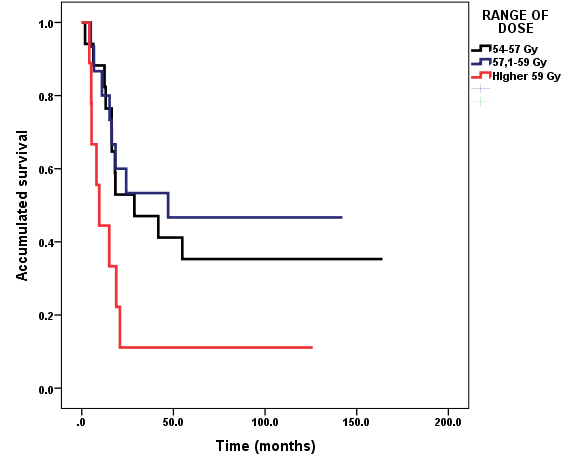
Figure 2 Survival according to range of dose. Diffuse intrinsic pontine brainstem gliomas in children and adolescents treated with Radiotherapy and Nimotuzumab.
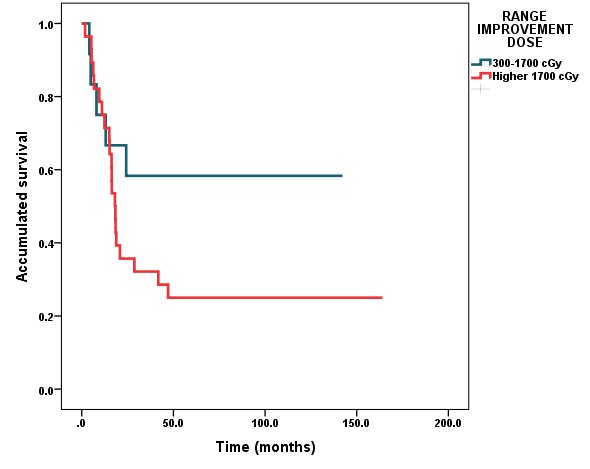
Figure 3 Survival according to range of improvement dose. Diffuse intrinsic pontine brainstem gliomas in children and adolescents treated with Radiotherapy and Nimotuzumab.
|
Age diagnostic |
Current age |
Sex |
KL |
RT dose Gy |
RT improvement |
Time survival |
Current activity |
|
9 |
23 |
male |
80 |
54* |
2400 |
164.1 |
University |
|
5 |
17 |
female |
60 |
57.4 |
1600 |
142.2 |
--- |
|
2 |
14 |
female |
70 |
55.8 |
1800 |
141.4 |
--- |
|
2 |
13 |
male |
50 |
57.6 |
1080 |
137.1 |
studying |
|
7 |
18 |
female |
50 |
59.4 |
1440 |
126.0 |
studying |
|
18 |
28 |
female |
50 |
57.4 |
1080 |
122.6 |
working |
|
5 |
15 |
female |
80 |
57.0 |
1440 |
119.4 |
studying |
|
2 |
12 |
female |
30 |
54.0 |
900 |
118.2 |
studying |
|
13 |
23 |
male |
80 |
57.6 |
2700 |
114.9. |
University |
|
6 |
15 |
female |
70 |
57.6 |
1260 |
106.7 |
studying |
|
10 |
19 |
male |
90 |
54.0 |
2200 |
104.5. |
studying |
|
15 |
23 |
male |
50 |
57.4 |
3200 |
99.7 |
--- |
|
16 |
22 |
female |
50 |
54.0 |
3200 |
68.6 |
University |
|
18 |
23 |
male |
80 |
54.0 |
2200 |
61.3. |
University |
|
|
|
* Re-irradiation 33.0 Gy |
|
|
|
||
Table 1 Characteristics and current activities of patients alive with long-term survival
Median overall survival from the beginning of treatment was 18.8 months (95%CI, 11.1-25.5); survival rates were 75,6 %, 41,3%, 36,6% and 34,1% at 1, 2, 3, 4 and 5 years respectively, estimated till 31 January 2021 (Figure 2). At present, 14 patients of the series are alive (34,1%). We were able to ascertain vital status of those patients who are alive at a minimum of 5 years, of them, 11 are incorporated to studies, including 4 who are attending studies at University.
Two patients were re-irradiated, one of them survival 9 years after that and is alive and studying at University, and the other improved with the re-irradiation, but died after 8 months.
Treatment was well tolerated and no grade 3 or higher grade toxicity was observed. The most frequent adverse event was alopecia in irradiation fields, observed in 35 patients (85,3%). Other reported adverse events were vomiting, head-ache, fever, tremor and nausea in less than 46 % of patients.
There were progression of the tumor in 2 patients, so they not completed the irradiation plan. In the 39 remaining patients there were clinical and imaging response in 36(92%).
Brainstem gliomas are an heterogeneous group of tumors occurring predominately in children, and generally they are diffuse intrinsic pontine gliomas (DIPG), with a peak between 6 and 7 years of age (6,8,40), primarly located in pons, infiltrating diffusely the pons (2,6,30,31) with a CT and MRI and clinical signs and symptoms that has been considered sufficient for diagnosis (1-9); because of the anatomical location of the tumor biopsy is risky and a surgical resection is not possible , but in the last years there is a tendency to do it (3,36,37,38).
Focal radiotherapy has been considered the standard treatment,1-3,5,8,35,37 offering certain benefits, but short-time response, with a bad prognosis and a median survival of one year or less, and few beyond 2 years.1-3,6,8-10,12,13,32,33,35,37,39 A radiation dose escalation does not improve outcome and neither chemotherapy nor hypofractionation have improved results.2,6,14,16-18,20,21
Nimotuzumab is an anti-EGFR humanized monoclonal antibody that blocks EGF, with anti-proliferative, antiangiogenic and pro-apoptotic activity,29 which explain the results observed in different reports.1,4,6,7,24,28,30,31-34 Nimotuzumab has demonstrated that does not produce toxicities, as commonly associated with other EGFR targeting agents, such as skin rash; condition that has allowed to perform combination with other drugs.1,4 Adverse events as alopecia, head-ache and nausea could be associated to the irradiation.6,32,33 Nimotuzumab combined with RT and continued after the period of irradiation for 1,2 or more years, was well tolerated , its prolonged use is safe and feasible,6,24,32,33 can be administered in outpatient settings,6 so permitting to attend school, or work. At this moment (Jan 2021), 11 patients of the Very Long Term Follow – up are in different levels of scholarship, of which 4 are attending at the University, only 1 patient of Very long-term follow-up have impairment of knowledge and are receiving special education.
Most patients experienced an improvement in neurological symptoms, it has been reported even in 80%.40 In 38 (96,7%) of our patients there were an improvement : in the group of Very Long Overall Survival, it appeared at a median dose of 1,700cGy. In the deceased group the median dose were improvement appeared was 1,980cgy, with not statistical significate difference. It has been suggest that low cumulative doses of RT, can provide neurological improvement.31,40
Rate survival results at long-term follow-up (76,6,%,46,3%,41,3%,36,1% and 34,1%) at 1,2,3,4 and 5 years, respectively, established till 10 or more years with the combined treatment of RT and the Nimotuzumab, were never reported previously. in DIPG.39 Jackson et al.35 reported only 2.6% at 5 or more years; Hoffman et al.5 in a Collaborative Report from the International and European Society for Pediatric Oncology DIPG Registries inform about 2,2% of survival at 5 years; Hassan et al.13 reported only 7,5% at 3 years. Crotty et al.,21 from the Seattle Children’s Hospital reported O% of OS at 5 year.
It’s important to remark that 36 patients (87,8%) received the Nimotuzumab doses programmed, with minor interruptions, so the anti-proliferative, anti-angiogenic and pro-apoptotic activity of the product29 were present during irradiation period and later during one, two or more years, and probably explain the very long overall survival.
When the disease progress, re-irradiation has been employed, and has been reported to give a certain survival benefit.1,41-43 In our 2 patients were re-irradiation was combined with Nimotuzumab, one is alive more than 5 years after the procedure, the other had a survival of 8 months, more than the survival benefit of 3,4 months reported.41,42
The association of RT and Nimotuzumab in the treatment of DIPG in children and adolescents is safe and feasible and could be a therapeutic option in order to increase very long-term survival. Re-irradiation is an option in case of relapse of tumor
None.
All authors declare that there is no conflict of interest.
None.

©2021 Alert, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.