International Journal of
eISSN: 2574-8084


Research Article Volume 8 Issue 2
1Faculty of Medicine, University of New South Wales, Australia
2Pittwater Day Surgery, Australia
3Neutral Bay Laser & Dermatology Clinic, Australia
4Northern Sydney Dermatology and Laser, Australia
5Genesis Care, Mater Hospital, Australia
Correspondence: Professor Gerald Blaise Fogarty, Genesis Care, St Vincent’s Hospital, Victoria St, Darlinghurst 2010, NSW, Australia, Tel +612-8302-5400, Fax +612-8302-5410
Received: March 15, 2021 | Published: April 7, 2021
Citation: Tighe DG, Tanous A, Flood J, et al. Lesion-based radiotherapy for non-melanoma skin cancer of the lower legs with a focus on radiation-induced ulcers. Int J Radiol Radiat Ther. 2021;8(2):44-54. DOI: 10.15406/ijrrt.2021.08.00293
Aim:Non-melanoma skin cancer (NMSC) of the lower legs is a challenge to treat. Surgery can be difficult given the challenged blood supply.Radiotherapy (RT) is a controversial treatment modality and some radiation oncologists (ROs) will not offer definitive treatment for lesions below the knee for fear of creating a radiation-induced ulcer. This study is a retrospective audit of a single RO’s treatment of lower leg NMSCs. The aim is to evaluate the efficacy of RT in gaining local control of these lesions. The aim is also to document the development of late side effects following RT, including radiation-induced ulcers and their treatment. Referral growth over time was also investigated.
Methods:Electronic medical records were searched for patients with lower leg NMSCs treated by the RO between January 2009 and December 2019 at three locations in Sydney, Australia (St Vincent’s Hospital, Mater Hospital, and Macquarie University Hospital). Patient, tumour, treatment, and outcome factors were collected and analysed.Referrals over time were recorded.
Results:111 lesions arising in 56 patients were identified. There was even distribution of sex and the mean age was 82 (range 57–95). There were 78 cutaneous squamous cell carcinomas (cSCCs) and 23 basal cell carcinomas (BCC). Median lesion size was 2 centimetres (range 1–10cm). The most common RT modality used was electrons (91 [82%]), followed by superficial RT (SXRT) (20 [18%]). Median duration of follow-up was 4 months (range 0–117 months). Of the 77 lesions treated with curative intent, cure was achieved in 74 (96%) lesions. 2 cSCCs and 1 BCC recurred, with a median time to recurrence of 24 months. 15 (14%) lesions developed a radiation-induced ulcer following RT. Median duration of therapy required for these ulcers was 5 months (range 1–55 months), with conservative treatment being the most common therapy used. Referrals increased from 8 in the 2008-2011 period to 26 in the 2016-2019 period.
Conclusion: This study showed RT treatment of lower leg NMSCs achieves local control of lesions with an acceptably low rate of radiation-induced ulcers, thus supporting the use of this modality for this patient population. Referrals grew over time which may reflect growing referrer knowledge and confidence in definitive RT below the knee.
Keywords: skin neoplasms, non-melanoma skin cancer, skin cancer, basal cellcarcinoma, squamouscellcarcinoma, radiotherapy, superficialradiotherapy, electrons, megavoltageradiotherapy, australia, legs, ulcers
Non-melanoma skin cancer (NMSC) is the most common cancer diagnosed in Australia.1 Expenditure on NMSC in Australia is second only to colorectal cancer.2The most common NMSCs are basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC).1
Surgical excision is seen as the ‘gold standard’ therapy for NMSC.3-7 However, surgery may not be suitable for all patients with NMSC, such as patients who are unfit for surgery due to age or medical comorbidities, or who have multiple lesions spanning a large area of skin. Surgery for lesions of the lower legs can be difficult given the challenged blood supply.In these situations, radiotherapy (RT) may be a preferred modality.8
RT for NMSC of the lower leg remains a controversial clinical scenario. Due to reduced vascularity inherent to the lower leg promoting poor wound healing, radiation can cause radiation-induced ulcers, particularly in patients with vascular disease, previous ulcers, or diabetes.9 Some radiation oncologists (ROs) will not offer definitive treatment for lesions below the knee for fear of creating a radiation-induced ulcer. Figure 1 shows an example of two radiation-induced ulcers on the lower legs. Reticence to use RT for lower leg NMSCs has created a paucity of high-quality research investigating if RT is an effective treatment option. Based on a Medline search current as of December 2020, there are no prospective trials comparing surgery and radiotherapy for lower leg NMSCs.
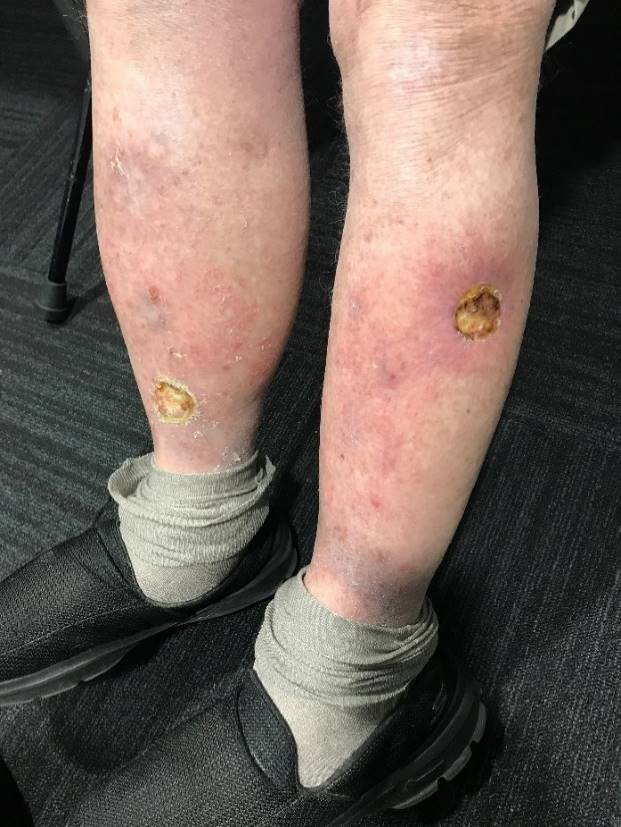
Figure 1 Radiation-induced ulcers on the lower legs following radiotherapy for non-melanoma skin cancers.
This study is a retrospective audit of a single radiation oncologist’s (RO) treatment of NMSC of the lower leg at Genesis Care sites at St Vincent’s Hospital, Mater Hospital, and Macquarie University Hospital in Sydney, Australia. The aim of this study was to evaluate the efficacy of RT in treating this patient cohort and any adverse effects arising from RT to the lower leg, with a particular focus on documenting incidents of radiation-induced ulcers.
This project was approved by the St Vincent’s Hospital Human Research Ethics Committee (application number 2019/ETH14034). Electronic medical records were searched for patients who had received radiotherapy for a NMSC lesion on the lower legs at the study locations between January 1 2009 and December 31 2019. Inclusion criteria required patients to be over 18 years of age and for their treatment to be prescribed by a single radiation oncologist (GBF). Lesions had to be below the tibial tuberosity and either biopsy-proven or diagnosed clinically as a NMSC by the referrer or RO. Treatment had to be lesion-based, not field based.
Patient, tumour, treatment, and outcome factors were collected for analysis. Patient factors included sex, age, referral date, immune status, and presence of existing peripheral vascular disease. Tumour factors included the type of non-melanoma skin cancer, the location of the lesion, macroscopic lesion size, and regional lymph node involvement. Treatment factors included the intent of treatment, radiotherapy modality used, and total radiation dose delivered.
Outcome factors included local recurrence of lesions, salvage therapies used for recurrent lesions, development of radiation-induced ulcers, therapies used for ulcers, and other late side effects of radiotherapy. Length of follow-up was determined according to the time between the end radiotherapy date and the date of the last correspondence either in or out of a patient’s medical record. Local recurrence was defined as recurrence of the lesion within the previous treatment field. Radiation-induced ulcers were defined as an ulcer that occurred within the RT treatment area after RT. If there was no mention of local recurrence or development of a radiation-induced ulcer in a patient’s medical records following radiotherapy until last follow-up, it was assumed that neither event occurred. This was regardless of the length of the follow-up.
All data was collated and analysed. During analysis of local recurrence, lesions treated with palliative intent were excluded due to the aim in a palliative setting being symptom control rather than local control.
138 patients were identified in the initial search through the electronic medical records. 82 patients were excluded due to either: having a tumour other than NMSC (24), RT not being given (13), duplicate files (13), field-based treatment (11), site of the lesion not below the tibial tuberosity (6), treatment prescribed by another RO (2), treatment occurring outside the study period (2), patient dying during treatment from an unrelated cause (1), and other reasons (10).
Patient factors
111 NMSCs on the lower legs across 56 patients were treated from January 1 2009 to December 31 2019. 29 patients had one lesion, 12 had two lesions, 9 had three lesions, 5 had four lesions, and one patient had 11 lesions (Table 1). 50% of patients were male and 50% were female. The mean age during treatment was 82 (range 57–95). 8 patients suffered from immunosuppression. The reasons for immunosuppression were treatments for autoimmune conditions (4), anti-rejection medication for transplant recipients (2), and therapies for haematological malignancies (2). 22 patients had existing vascular disease of their lower legs. Reasons for this were lymphoedema (11), often as a complication from prior surgery such as groin dissection, peripheral vascular disease (6), vascular complications of diabetes (4), and bilateral deep vein thromboses (1). Referrals increased from 8 in the 2008-2011 period to 26 in the 2016-2019 period (Figure 2).
Lesions per patient |
||
1 |
29 |
52 |
2 |
12 |
21 |
3 |
9 |
16 |
4 |
5 |
9 |
11 |
1 |
2 |
Sex |
||
Male |
28 |
50 |
Female |
28 |
50 |
Age at treatment(years) † |
||
Mean |
82 |
|
Range |
57 – 95 |
|
Referrals per period |
||
2008-2011‡ |
8 |
14 |
2012-2015 |
22 |
39 |
2016-2019 |
26 |
47 |
Immune statusbefore or aftertreatment |
||
Immunocompetent |
48 |
86 |
Immunosuppressed |
8§ |
14 |
Vasculardisease of the lower legs |
||
No existingvasculardisease |
34 |
61 |
Existingvasculardisease |
22¶ |
39 |
Table 1 Patient characteristics (n=56)
† If a patient had multiple lesions treated, the age at their first treatment was used
‡ Referral to the radiation oncologist was made in 2008, but treatment was given after 1/01/2009
§Autoimmune conditions=4; Transplant recipient=2; haematological malignancy=2
¶ Lymphoedema=11; peripheral vascular disease=6; vascular complications of diabetes=4; bilateral deep vein thromboses=1
Tumour factors
111 lesions were treated, with cSCC being the predominant type of NMSC (n=78), followed by BCC (n=23; Table 2). 71 lesions were biopsy-proven, with the remaining 40 being diagnosed clinically. 61 lesions were on the left lower leg and 50 were on the right. The most common location of lesions was on the lower lateral leg (28 [25%]), followed by the middle lateral leg (24 [22%]). 7 lesions (6%) were on the dorsum of the foot. Median macroscopic lesion size was 2 centimetres (range 1–10cm), though lesion size was unavailable for 57 lesions. T stage10 was T1 for 28 lesions, T2 for 16 lesions, and T3 for 10 lesions. 2 patients (4%) had regional lymph nodes involved.
Variable |
Number |
Percentage (%) |
|
Type of NMSC |
|||
cSCC |
78 |
70 |
|
BCC |
23 |
21 |
|
cSCC in situ |
5 |
5 |
|
Basosquamous |
1 |
1 |
|
Unknown |
4 |
3 |
|
Site |
|||
Left or right |
|||
Left |
61 |
55 |
|
Right |
50 |
45 |
|
Location on lowerleg |
|||
Uppermedial |
12 |
11 |
|
Upperlateral |
7 |
6 |
|
Middle medial |
11 |
10 |
|
Middle lateral |
24 |
22 |
|
Lowermedial |
22 |
20 |
|
Lowerlateral |
28 |
25 |
|
Foot |
7 |
6 |
|
T stage |
|||
T1 (≤ 20mm) |
28 |
25 |
|
T2 (20mm to ≤40mm) |
16 |
14 |
|
T3 (>40mm) |
10 |
9 |
|
Unknown |
57 |
52 |
|
Macroscopiclesion size (centimetres) |
|||
1 – 1.99 |
8 |
7 |
|
2 – 2.99 |
23 |
20 |
|
3 – 3.99 |
10 |
9 |
|
4 – 4.99 |
3 |
3 |
|
5 – 5.99 |
5 |
4 |
|
6 – 6.99 |
2 |
2 |
|
7 – 7.99 |
0 |
0 |
|
8 – 8.99 |
2 |
2 |
|
9 – 9.99 |
0 |
0 |
|
10 – 10.99 |
1 |
1 |
|
Unknown |
57 |
52 |
|
Median |
2 |
||
Range |
1 – 10 |
||
Regionallymphnodes (n=56 patients) |
|||
Not involved |
54 |
96 |
|
Involved |
2 |
4 |
|
Table 2 Tumour characteristics (n=111)
NMSC, non-melanoma skin cancer; cSCC, cutaneous squamous cell carcinoma; BCC, basal cell carcinoma
Treatment factors
Treatment intent was definitive for 73 lesions (65%), adjuvant for 4 lesions (4%), and palliative for 34 lesions (31%; Table 3). Electron treatment, usually 6MeV, was the most common modality used (91 [82%]), followed by superficial x-ray radiation therapy (SXRT; 20 [18%]). Figure 3 shows a patient receiving SXRT using an Xstrahl SXRT machine. The most common fractionation patterns used for lesions treated with curative intent (either definitive or adjuvant) were 50 Gy/25 fractions (13 [17%]), 40/10 (8 [11%]), and 50/20 (7 [9%]). 6 lesions treated with curative intent had treatment ceased early due to already achieving adequate skin reaction consistent with cure as judged by the prescribing RO. 8 lesions required extra doses than the initial plan due to lack of adequate skin reaction. 29 lesions were planned for adaptive split course radiotherapy (ASCRT), a technique where a first phase of RT is given to reduce the volume of a lesion, followed by a second phase of RT after an 8-week treatment break.11 ASCRT is a relatively high dose treatment given with palliative intent but often leads to complete response (CR). In our study, 7 lesions treated with ASCRT did not require the second phase due to CR after phase one of treatment.
Variable |
Number |
Percentage (%) |
|
Treatmentintent |
|||
Definitive |
73 |
65 |
|
Adjuvant |
4 |
4 |
|
Palliative |
34 |
31 |
|
Modality |
|||
Electrons |
91 |
82 |
|
SXRT |
20 |
18 |
|
Radiation fractionation pattern (Gray/number of fractions) |
|||
Lesionstreatedwithdefinitive or adjuvant intent (n=77) |
|||
24/4 |
3 |
4 |
|
24/8 |
1 |
1 |
|
25/10 |
2 |
3 |
|
30/5 |
1 |
1 |
|
36/6 |
1 |
1 |
|
36/18 |
1 |
1 |
|
40/10 |
8 |
11 |
|
44/22 |
1 |
1 |
|
45/15 |
2 |
3 |
|
45/20 |
3 |
4 |
|
45/25 |
4 |
5 |
|
48/12 |
1 |
1 |
|
48/24 |
3 |
4 |
|
50/20 |
7 |
9 |
|
50/25 |
13 |
17 |
|
55/25 |
3 |
4 |
|
56/28 |
1 |
1 |
|
60/20 |
1 |
1 |
|
60/30 |
3 |
4 |
|
Non-standard fractionation patterns |
16 |
21 |
|
Unknown |
2 |
3 |
|
Lesionstreatedwith palliative intent (n=34) |
|||
20/5 |
1 |
3 |
|
25/10 |
5 |
14 |
|
25/5 |
3 |
9 |
|
36/6 |
2 |
6 |
|
40/10 |
3 |
9 |
|
45/10 |
3 |
9 |
|
45/15 |
4 |
12 |
|
45/20 |
1 |
3 |
|
50/10 |
1 |
3 |
|
50/15 |
1 |
3 |
|
50/20 |
6 |
17 |
|
60/30 |
2 |
6 |
|
Non-standard fractionation patterns |
2 |
6 |
|
Table 3 Treatment characteristics (n=111)
SXRT, superficial x-ray radiation therapy
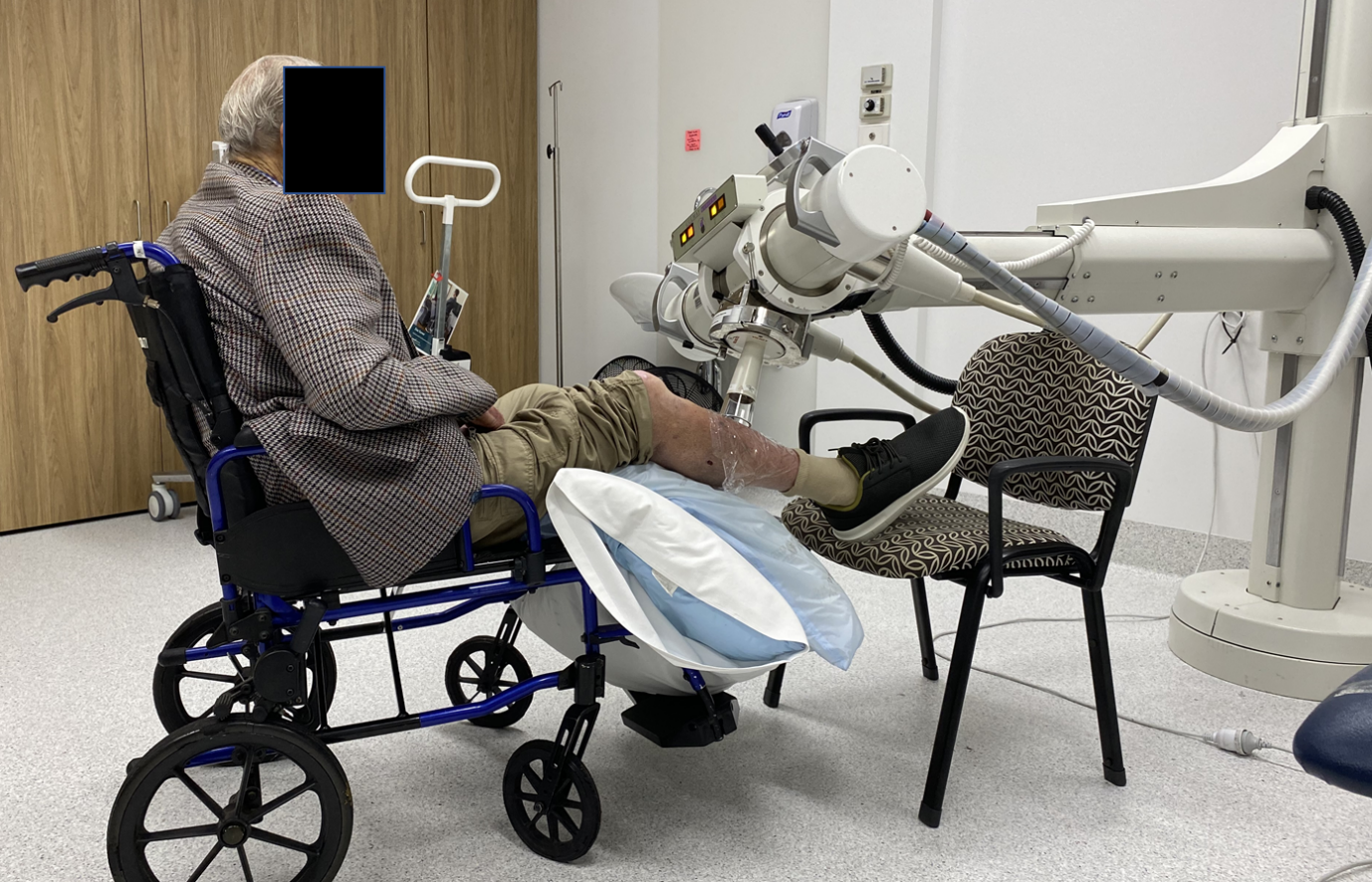
Figure 3 Leg treatment showing ease of set up with Xstrahl SXRT machine. Wheelchair bound patient having SXRT treatment to lesion on right lower leg. Ease of set up enables quick treatment without need for transfers from the chair to bed, minimising discomfort for patients and making workflow smooth and more efficient.
Outcome factors
Median follow-up duration was 4 months (range 0–117months).
Of the 73 lesions treated with definitive intent, 2 lesions (3%) recurred within the previous treatment field as at September 2020 (Table 4 and Table 5). Median time to recurrence was 26 months (range 3–49 months). The first recurrence occurred in an 86-year-old immunocompetent male with a cSCC on his right lower medial leg. The recurrence was diagnosed on biopsy and successfully salvaged with surgery. The second recurrence occurred in a 71-year-old immunocompetent female with a cSCC on her left lower medial leg. The recurrence was diagnosed clinically and successfully salvaged with radiotherapy.
Variable |
Number |
Percentage (%) |
|
RECURRENCE |
|||
Definitivelesions(n=73) |
|||
Local recurrence |
|||
Yes |
2 |
3 |
|
No |
71 |
97 |
|
Time to recurrence (months) |
|||
Mean |
26 |
||
Median |
26 |
||
Range |
3 – 49 |
||
Method of diagnosingrecurrence |
|||
Clinical |
1 |
||
Biopsy |
1 |
||
Salvage therapyused |
|||
Radiotherapy |
1 |
||
Surgery |
1 |
||
Adjuvant lesions (n=4) |
|||
Local recurrence |
|||
Yes |
1 |
25 |
|
No |
3 |
75 |
|
RADIATION-INDUCED ULCERS |
|||
Radiation-inducedulcerdeveloped |
|||
Yes |
15 |
14 |
|
No |
96 |
86 |
|
Duration of therapy for ulcer (months) |
|||
Mean |
9.6 |
||
Median |
5 |
||
Range |
1 – 55 |
||
Treatment for ulcer |
|||
Conservative |
12 |
||
Surgery |
2 |
||
Hyperbaricoxygen |
1 |
||
Table 4 Oncological and Ulcer Outcomes
Median duration of follow-up=4 months (range 0 – 117 months)
|
PATIENT FACTORS |
TUMOUR FACTORS |
TREATMENT FACTORS |
OUTCOMES |
|
||||
Lesion |
Age at treatment, sex, immunocompetent (IC) or immunosuppressed (IS) |
Existingvasculardisease of the lower legs |
Site, histological type, T stage |
Regionallymphnodeinvolvement |
Treatmentmodality*, total dose (Gy)/fractions |
Time to recurrence (months) |
Salvage therapyused |
Did salvage therapyresolve the recurrence? |
Comment |
DEFINITIVE |
|||||||||
1 |
86, M, IC |
Poor vascularsupply |
Lowermedial, cSCC, unknown |
No |
Electrons, 56/28 |
49 |
Surgery |
Yes |
Treatmentceasedearly due to significant desquamation |
2 |
71, F, IC |
No |
Lowermedial, cSCC, T2 |
No |
Electrons, 63/30 |
3 |
Radiotherapy |
Yes |
Extra fractions given due to inadequate skin reaction |
ADJUVANT |
|||||||||
3 |
76, M, IC |
No |
Uppermedial, BCC, unknown |
Yes – inguinal nodes |
Electrons, 60/30 |
24 |
Surgery |
No – furtherradiotherapyrequired |
|
Table 5 Recurrent lesions
cSCC, cutaneous squamous cell carcinoma; BCC, basal cell carcinoma
*Most electron treatments were 6MeV
Of the 4 lesions treated in the adjuvant setting, 1 lesion (25%) recurred. The recurrence occurred in a 76-year-old immunocompetent male with a BCC on his left upper medial leg. The BCC had been surgically excised but had recurred. Lymphadenopathy in the inguinal region was detected at presentation to the RO. Adjuvant RT was given but the lesion again recurred 24 months later, following which an above-knee amputation was performed to ensure local control due to the aggressive nature of disease. Further RT was given to the left leg stump and lower abdomen for symptomatic recurrence of the BCC.
Two patients had regional lymph nodes involved prior to RT. The first patient was an 85-year-old male with four clinically diagnosed cSCCs on his right lower leg (three on the upper medial lower leg and one on the lower medial lower leg). He had existing poor vascularity of the lower legs and was immunocompetent. There was lymphadenopathy detected in the right popliteal fossa. He was treated with palliative intent using an electron modality and received a dose of 45 Gy in 15 fractions for each lesion. The patient had 80 months of follow-up, during which the lesions did not locally recur, no radiation-induced ulcer developed, and no late side effects were seen.
The second patient with regional lymph node involvement is the 76-year-old male who developed recurrent BCC after adjuvant RT described above.
Only 1 lesion developed a late side effect after radiotherapy, defined as an adverse effect with onset after 6 months of the end of RT.12 The late side effect was a rash, which was biopsied under suspicion of malignancy, but no malignancy was found. The patient was referred back to her dermatologist for follow-up.
Radiation-induced ulcers
15 lesions (14%) developed a radiation-induced ulcer following radiotherapy treatment (Table 6). Figure 4 depicts the distribution of the ulcers. The most common location for ulcers to develop was the lower lateral zone of the leg (7/15 [47%]), followed by the middle lateral (4/15 [27%]). 5/15 ulcers (33.3%) developed in patients who were immunosuppressed. This equates to 2/8 (25%) immunosuppressed patients developing ulcers, as one of these patients had 4 lesions treated. 3/15 ulcers (20%) developed in patients with existing vascular disease, equating to 3/22 (14%) patients with known vascular disease developing ulcers.
|
PATIENT FACTORS |
TUMOUR FACTORS |
TREATMENT FACTORS |
OUTCOMES |
|
||
Lesion |
Age at treatment, sex, immunocompetent (IC) or immunosuppressed (IS) |
Existingvasculardisease of the lower legs |
Site, histological type, T stage |
Treatmentmodality*, total dose (Gy)/fractions |
Duration of ulcer (months) |
Treatmentused |
Comment |
DEFINITIVE |
|||||||
1 |
79, F, IS |
Yes – legswelling |
Lowermedial, cSCC, T2 |
Electrons, 36/18 |
6 |
Dressings, antibiotics |
Initial prescription was 50/25 but treatmentceasedearly due to pain, tenderness, and woundexudate |
2 |
85, M, IC |
No |
Lowerlateral, cSCC, T1 |
Electrons, 56/10 |
1 |
Dressings |
|
3 |
90, F, IC |
No |
Lowerlateral, BCC, T1 |
Electrons, 44/22 |
8 |
Dressings |
|
4 |
69, M, IC |
No |
Middle lateral, BCC, T3 |
Electrons, 57.5/23 |
1 |
Dressings |
|
5 |
85, F, IC |
No |
Middle medial, BCC, unknown |
Superficialradiotherapy, unknown |
23 |
Dressings |
|
ADJUVANT |
|||||||
6 |
72, F, IC |
No |
Lowermedial, cSCC, T2 |
Electrons, 50/25 |
16 |
Hyperbaricoxygen |
|
7 |
82, F, IC |
No |
Lowerlateral, cSCC, unknown |
Electrons, 60/30 |
55 |
Pentoxifylline/vitamin E ointment, hyperbaricoxygen, stem cellproducts |
|
PALLIATIVE |
|||||||
8 |
92, F, IC |
Yes – chroniclymphoedema |
Lowerlateral, cSCC, T3 |
Electrons, 50/20 |
6 |
Dressings |
|
9 |
92, F IC |
Yes – stent in leftleg |
Middle lateral, cSCC, unknown |
Electrons, 25/5 |
2 |
Dressings |
Adaptive split course radiotherapyplanned, but second phase not required |
10 |
88, F, IS |
No |
Lowerlateral, cSCC, unknown |
Superficialradiotherapy (phase 1), electrons (phase 2), 45/10 |
5 |
Surgery |
Adaptive split course radiotherapyused |
11 |
88, F, IS |
No |
Lowerlateral, cSCC, unknown |
Superficialradiotherapy (phase 1), electrons (phase 2), 45/10 |
5 |
Dressings |
Adaptive split course radiotherapyused |
12 |
88, F, IS |
No |
Lowerlateral, cSCC, unknown |
Superficialradiotherapy (phase 1), electrons (phase 2), 45/10 |
5 |
Dressings |
Adaptive split course radiotherapyused |
13 |
88, F, IS |
No |
Middle lateral, cSCC, unknown |
Superficialradiotherapy (phase 1), electrons (phase 2), 45/10 |
5 |
Surgery |
Adaptive split course radiotherapyused |
14 |
92, M, IC |
No |
Middle lateral, cSCC, T3 |
Superficialradiotherapy, 36/6 |
3 |
Dressings |
Adaptive split course radiotherapyused |
15 |
95, F, IC |
No |
Lowermedial, baso-squamous, unknown |
Electrons, 50/20 |
3 |
Antibiotics, surgery |
|
Table 6 Radiation-induced ulcers
cSCC, cutaneous squamous cell carcinoma; BCC, basal cell carcinoma
*Most electron treatments were 6MeV
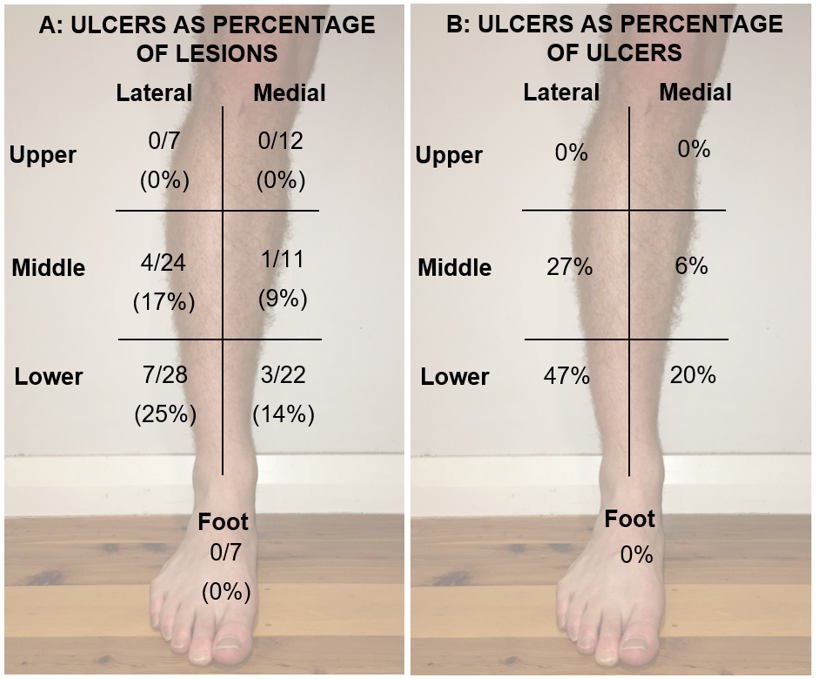
Figure 4 Distribution of radiation-induced ulcers following radiotherapy to lower leg NMSC by anatomical zones. The leg was divided into 6 zones. They are, for both medial and lateral, the upper third, middle third and lower third zones.
In each zone in A, the top line shows absolute number of ulcers over the number of lesions treated in each zone; the bottom line shows this as a percentage in brackets. In each zone in B, the number represents the number of ulcers that developed in each zone as a percentage of all the ulcers in the series. For example, in the lateral lower zone, A shows that 7 lesions treated out of 28 developed an ulcer (25%), and B shows that the same zone was where 47% (7/15) of the ulcers occurred.
The median duration of therapy required for an ulcer was 5 months (range 1–55 months). 12 ulcers were treated with conservative treatment, which involved dressings and oral and topical antibiotics. 2 ulcers received surgical therapy, and 1 ulcer was treated with hyperbaric oxygen. All radiation-induced ulcers resolved after their respective therapies.
In this retrospective audit of a single RO’s treatment of lower leg NMSC, 97% of lesions treated with definitive RT were locally controlled at last follow-up. Only 2 lesions (3%) recurred, with a median time to recurrence of 26 months. Both recurrent lesions were cSCCs. In the lesions receiving adjuvant RT, only 1 lesion, which was a BCC, recurred. Of the 111 lesions treated, 15 (14%) developed a radiation-induced ulcer. Most ulcers were treated conservatively with dressings and antibiotics, and all were eventually cured. There was no case where there was a recurrence and an ulcer, showing that NMSCs of the lower leg in this series were radiosensitive.
In Roth et al’s retrospective review of 151 lower leg BCCs and cSCCs treated with superficial radiotherapy, RT yielded a local control rate of 97.4%.13Our study showed a similar rate of local control. Roth et al’s review only included lesions treated with superficial radiotherapy, whereas in our study electron treatment was the most common RT modality. The fact that in both studies the local control rate was similar demonstrates that RT should be considered a useful option in treating lower leg NMSCs as different modalities can be used to suit patient’s circumstances without compromising oncological outcomes.
In a retrospective analysis of 65 BCCs and cSCCs on the lower leg treated with RT, Podd found an overall recurrence rate of 4.6%.14 Our study is again consistent with this result, reinforcing that RT is an effective option for lower leg NMSCs. 9.6% of lesions within Podd’s study developed into radiation-induced ulcers following RT. Our study reported a slightly higher rate of radiation-induced ulcers of 14%. This may be due to our inclusion of 22 patients with existing vascular disease of whom 3 developed ulcers, whereas Podd’s review excluded these patients.
Cox and Dyson also conducted a retrospective review of lower leg NMSCs treated with RT.15They reviewed 141 lesions and found that 33% of lesions exhibited poor healing. This is significantly higher than the rate of radiation-induced ulcers in our study, however comparison between the two studies is challenging for two reasons. Firstly, ‘poor healing’ in Cox and Dyson’s study was defined as either residual ulceration or cessation of a reduction in diameter of a tumour, hence does not correlate accurately with development of radiation-induced ulcers as measured in our study. Furthermore, 42% of lesions within this study were Bowen’s disease (cSCC in situ), whereas this lesion type comprised only 5% of tumours in our study. Differing rates of healing between the two studies could be confounded by differences in tumour types. Table 7 compares the results of our study with those of Roth et al.,13 Podd,14 and Cox and Dyson.15
|
Number of non-melanoma skin cancers included in study |
Recurrence rate (%) |
Ulceration rate (%) |
Roth et al [13] |
151 |
2.6 |
Not measured |
Podd[14] |
65 |
4.6 |
9.2 |
Cox and Dyson [15] |
141 |
0† |
33‡ |
Our study |
111 |
4§ |
14 |
Table 7 Comparison of studies of radiotherapyforlowerleg non-melanoma skin cancers
†Local recurrence rates were only reported for Bowen’s disease (cSCC in situ) treated with radiotherapy (n=59)
‡Represents residual ulceration or cessation of a reduction in diameter of a tumour
§Local recurrence rate is only for lesions treated definitively or in the adjuvant setting (n=77)
When documenting the location of lesions, the lower leg was divided into six zones and the foot. This was to capture which zones of the leg may be at most risk of developing a radiation-induced ulcer if RT is given. The zones used reflect the venous drainage of the lower leg, given that venous disease is a common risk factor for lower leg ulcer development.16The medial and lateral division stems from the drainage medially by the great saphenous vein and laterally by the small saphenous vein. Similarly, the venous drainage of the foot differs from that of the rest of the lower leg.17The division into upper, middle, and lower reflects the increasing likelihood for ulcer formation towards the ankle seen in other pathophysiological processes. For example, venous ulcers are most likely to develop above the medial or lateral malleoli (the ‘gaiter’ area), indicating poor venous drainage in this area.16
The most common location for development of radiation-induced ulcer in our study was the lower lateral area. This location is a common area for ulcer formation from other causes such as venous insufficiency.16This finding suggests that ROs should treat lower leg NMSCs in the lower lateral area with more caution, especially if there is pre-existing vascular disease, diabetes or immunosuppression. Of the 8 patients who were immunosuppressed, only 2 of them developed radiation-induced ulcers. Immunosuppression is a well-recognised risk factor for poor wound healing,18 however our findings suggest that it is not an absolute reason for patients not to receive RT for their lower leg NMSCs as the majority may not develop a radiation-induced ulcer. Of the 22 patients with known vascular disease, only 3 developed radiation-induced ulcers. Whilst vascular disease is a risk factor for lower leg ulcers,16our study suggests that RT can be given safely for some patients with lower leg NMSCs. Due to its retrospective nature, our study cannot be used to predict which patients with immunosuppression or vascular disease can be given RT safely, however this could be achieved in future prospective trials.
This study also reported the rate of referrals to the skin cancer RT service over time. The rate of referral increased from 8 in the 2008-2011 period to 26 in the 2016-2019 period. This increase may suggest that referring dermatologists are increasingly aware of the value of RT for NMSCs on the lower leg. This may also be a result of more proactive education, as described by Fogarty et al.19 Increasing referrals to RT have also been seen in NMSCs in other locations, such as nose lesions,20 suggesting RT’s popularity in treating skin cancer is increasing.
There are significant limits to this study. Our study is limited by the assumption that if a patient was not referred back following the end of RT, then this meant there was no recurrence. A patient could have developed a local recurrence but presented to another clinician who did not report this to the treating RO. Lack of histopathological diagnosis for some lesions also weakens our data. This resulted from biopsies being either declined or not possible in some cases due to fear of bleeding and ulcer formation due to pre-existing vascular issues. Being a retrospective review, data missing from medical records precluded a more complete data set.
Our retrospective audit showed RT to be an effective option for treating NMSCs of the lower leg. Local control was achieved in most lesions and the rate of development of radiation-induced ulcers was acceptably low as compared to what is commonly thought, and all ulcers eventually healed with appropriate care. Both findings are consistent with similar studies in the field. Given the current paucity of high-quality evidence for RT of lower leg NMSCs, future prospective, randomised trials comparing RT with other treatment modalities for this patient population are needed. Our study may hopefully inform the development of such future trials.
This retrospective audit of 111 NMSCs on the lower legs treated with RT showed a local control rate of 74/77 (96%) for lesions treated with curative intent. Radiation-induced ulcers occurred in only 15/111 (14%) lesions treated. This study therefore supports the safe use of RT for these lesions and may help guide the development of future prospective trials comparing RT with other modalities for this patient cohort.
The study team acknowledges the support of the Genesis Care team at St Vincent’s, Mater, and Macquarie University Hospitals in Sydney, Australia, and the patients and carers who made this study possible. We also wish to gratefully acknowledge Xstrahl Pty Ltd for funding the open access publication.
We wish to gratefully acknowledgeXstrahl Pty Ltd for funding the open access publication.
The authors declare that there is no conflict of interest.

©2021 Tighe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.