International Journal of
eISSN: 2574-8084


Retrospective Study Volume 11 Issue 3
1Genesis Care, Australia
2Sun Doctors Skin Cancer Clinics, Queensland, Australia
3Clinic Skin, Queensland, Australia
4On Point Skin Cancer Clinic, Queensland, Australia
Correspondence: Bradley Wong, Genesis Care, 10 King Street, Buderim, Queensland, Australia
Received: May 31, 2024 | Published: June 14, 2024
Citation: Wong B, Webb S, Powell M, et al. Keratinocyte cancer of the lower limb in the frail elderly – acceptable results for 96 lesions treated with a shortened radiotherapy protocol. Int J Radiol Radiat Ther. 2024;11(3):60-65. DOI: 10.15406/ijrrt.2024.11.00387
Keratinocyte cancer (KC) is the most prevalent cancer globally, with many patients developing multiple lesions as they age. Surgery is not practical for all patients, particularly older individuals with comorbidities such as vascular insufficiency or anticoagulation, which can be particularly challenging in anatomic locations such as the lower limbs. New radiotherapy (RT) technology and protocols have improved outcomes for patients by minimising toxicity whilst maintaining efficacy. An innovative RT protocol for KC treatment was developed to address this, definitive Adaptive Split Course Radiotherapy (ASCRT), in which the RT course is divided into two phases separated by an extended mid-treatment break with the aim of minimising toxicity. This study investigated the efficacy, safety, and patient satisfaction in a cohort of 47 patients from Queensland, Australia, who had 96 KC or symptomatic precancerous lesions on the lower limbs treated with a modified ASCRT protocol. Out of the complete cohort, 63 lesions that received both phases, the complete response rate was 98.4% (62/63), with only one grade 3 toxicity. Six patients developed an ulcer after treatment yielding an in-field ulcer rate of 6.6% (6/91), which resolved within 12-months. These results demonstrate that ASCRT is a viable treatment alternative for high-risk patient populations that potentially reduces toxicity without compromising efficacy. This protocol can potentially be expanded to other anatomic sites where there are concerns for the tolerability of a standard radiotherapy course.
Keywords: Radiotherapy, lower limbs, elderly, keratinocyte cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma external beam, electrons split course
RT, radiotherapy; KC, keratinocyte cancer; ASCRT, adaptive split course radiotherapy; BCC, basal cell carcinoma; SCC, squamous cell carcinoma; QoL, quality of life; 3DCRT, 3D conformal radiation therapy; IMRT, intensity modulated radiation therapy; VMAT, volumetric modulated arc therapy; CTCAE, common toxicity criteria adverse events; Gray (Gy); CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PVD, peripheral vascular disease; HSK, hyperkeratotic solar keratoses; IEC, Intraepidermal Carcinoma; KA, keratoacanthoma; ECOG, eastern cooperative oncology group
Keratinocyte cancer, namely Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC), represent the overwhelming burden of dermatological malignancies, with a rising incidence due to UVL exposure and aging populations.1-3 Queensland has the highest BCC and SCC incidence in the world1 and suffers from the tyranny of distance in that a fractionated course of RT is burdensome for patients who live a substantial distance from treatment centres. Advanced age and comorbidities often influence the choice and delivery of treatment, as well as patient outcomes.4-6 Many patients with symptomatic locally advanced skin cancers that would benefit from RT are frail and/or elderly, which often further precludes the ability to attend a protracted treatment course. Furthermore, prior KC incidence increases the risk of future lesions,7,8 meaning these patients often require frequent episodes of care.
While surgery is effective, it can be disfiguring, require complex aftercare and can be particularly complicated in the elderly and/or in those with comorbidities.9,10 Furthermore, surgery to the lower limbs is often difficult due to vascular insufficiency and/or tight skin necessitating invasive skin grafts and flaps, and extended periods of post-operative immobilisation/elevation. Frequent surgical intervention may not be practical for patients with high disease burdens who may require multiple treatments annually. Such individuals often develop surgical fatigue and seek out alternative non-invasive treatment options. Although RT has been used to successfully treat skin cancer for over 100 years,11 it is often underutilised12 despite recommendations for patients who refuse or are unsuitable for surgery.13 RT is also used effectively to improve Quality of Life (QoL) in the palliative setting.14
Although non-invasive, RT can disrupt the regenerative capacity of the skin, thereby risking delayed healing or other issues.15 Like surgery, healing complications are more common on the lower limbs. 16-18 ASCRT, or the Fogarty protocol, was developed with the goal of improving compliance, safety, and tolerability, whilst maintaining efficacy.19,20 ASCRT involves dividing the standard course into two distinct phases with an extended mid-treatment break to mitigate toxicity, whilst traditionally delivered in fewer overall fractions.
Building on previous case studies, we have used the ASCRT protocol to treat 96 lower limb BCC, SCC, and symptomatic pre-cancerous lesions in 47 patients who were unsuitable for or declined surgery and/or for whom there were concerns about tolerability of a standard RT course.
This retrospective analysis was performed on patients treated by a single radiation oncologist across four GenesisCare centres in Queensland, Australia. Patient treatment planning and follow-ups were managed by the radiation oncologist as well as the local treatment team that included radiation therapists and radiation-specialist nurses. If patients were unable to return for final assessments specific to this study, telephonic assessments were performed where possible by the treating clinician.
Treatment protocol
Patients presenting with lower-limb BCC, SCC, or symptomatic precancerous lesions were prescribed the ASCRT protocol 20 if they were deemed unsuitable for a standard course of radiotherapy due to advanced age and/or comorbidities and difficulty attending a fully fractionated course. Lower limb was defined as below the tibial tuberosity. Exclusion criteria included overlap with previous radiotherapy, nodal involvement, field-based therapy, and treatment with palliative intent.
The ASCRT protocol consists of 25 Gray (Gy) delivered in 10 daily fractions (2.5 Gy / fraction) weekdays over 2 weeks. A mid-treatment break of 8-weeks was scheduled followed by the patient returning for lesion assessment. If deemed appropriate based on a combination of tumour response, first phase tolerability and patient comorbidities, then a second phase commenced consisted of 20 Gy delivered in 10 daily fractions (2 Gy / fraction) delivered on weekdays over 2-weeks. Unlike the original description of ASCRT, for this cohort there was no change to the planned volume even if a reduction in cancer volume was apparent. This was partially to staff limitations and the need to avoid replanning to reduce unnecessary time for patients in clinic.
The ASCRT was modified from the previous protocol reported by Fogarty et al19,20 (25 Gy / 5 fractions, 8 - week break, 20 Gy / 5 fractions) as all the lesions in this study were treated on the lower limb and there was concern about hypofractionation. Similar to the Fogarty protocol, the first phase was aimed to deliver a dose of RT that would be just below that which would give troublesome acute RT side effects. The break would allow the acute toxicities from the first phase to resolve, decreasing the overall toxicity experienced at the end of the second phase compared with the acute toxicity experienced from a full continuous course of RT.19 In this study, the approach was to treat the lesions only.
Each treatment was prepared using 3D treatment planning system Eclipse or Monaco. Bolus of 0.8 cm-1cm was employed to maintain maximal dose at skin level. The approach was to treat the lesions only. Lesions were treated with electrons, 3D Conformal Radiation Therapy (3DCRT), Intensity Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT), delivered using Varian Truebeam, Varian iX, or Elekta Versa HD linear accelerators. Variable sized planning margins were applied to account for subclinical spread based on tumour characteristics
Toxicity and cosmesis
Patients were evaluated for skin integrity and suitability for treatment by nurses prior to commencing treatment each day. Toxicity was assessed using the Common Toxicity Criteria Adverse Events (CTCAE) v 5.0 21 by medical and nursing staff prior to commencing the second phase of treatment to confirm suitability to continue treatment. Cosmesis was assessed by the treating radiation oncologist at final follow-up using the Lovett’s cosmesis scale.22
Lesion response
Treatment response was assessed using the RECIST criteria, grading lesions as complete response (CR), partial response (PR), progressive disease (PD), or stable disease (SD).
Patient satisfaction
At final follow, patients were assessed on their experience of radiotherapy for management of their skin lesion. Patients were asked the following questions:
1) Has this treatment improved your QoL – yes/no
2) Would you have further radiotherapy to control skin cancers – yes/no/I don’t know
Statistics
Data analysis was performed using descriptive statistics to summarize patient demographics, lesion characteristics, and treatment outcomes.
Ethics
Human Research Ethics Approval was granted by St Vincent's Hospital Human Research Ethics Committee for the retrospective analysis of this cohort (2022/ETH00247).
Demographic
Forty-seven patients, 25 male / 22 Female (median age 83 years; range 67-96), (Table 1) who were prescribed modified ASCRT to treat KC lesions on the lower limbs were retrospectively analysed. Relevant comorbidities (Table 1) included, immunosuppression (5/47), diabetes (6/47), peripheral vascular disease (PVD) (7/47) and smoking – current or reformed (18/47). Patients were predominantly ECOG 1 and 2 (Table 1). A total of 96 KC or symptomatic pre-cancerous lesions (56 SCC, 25 BCC, 5 Hyperkeratotic Solar Keratoses / HSK, 5 Intraepidermal Carcinomas / IEC, 4 Keratoacanthomas / KA, 1 Basosquamous) (Table 2) were treated. Wherever possible, biopsy confirmation was performed in 58.3% (56 / 96), otherwise clinical assessment was made in consultation with the referring clinician. Patients with multiple similar lesions commonly underwent biopsy of only a single representative lesion. Other patients who did not receive biopsy had previously received histological confirmation of similar lesions in close proximity, refused biopsy, or the referring clinician deemed biopsy to be unsafe due to poor healing and/or infection risk. 78.1% (75 / 96) were < 2 cm in diameter, while 15.6% (15 / 96) and 6.35% (6 / 96) were 2 -5 cm and > 5 cm in diameter, respectively (Table 2). All patients had at least one other KC treated previously, with 30% (14 / 47) having received RT to at least one previous skin cancer.
|
Characteristic |
Category |
Overall |
|
Number |
|
N = 47 |
|
Age (years) |
|
82.47 (Mean) (SD 6.57) |
|
|
|
83 (Median) (range: 67, 96) |
|
|
|
67, 96 |
|
|
|
n (%) |
|
Gender |
Male |
25 (53%) |
|
|
Female |
22 (47%) |
|
ECOG |
0 |
4 (8.5%) |
|
|
1 |
21 (44.7%) |
|
|
2 |
19 (40.4%) |
|
|
3 |
3 (6.4%) |
|
Co-morbidity / Other factor |
Immunosuppression |
5 (10.6%) |
|
|
Diabetes |
6 (12.8%) |
|
|
PVD |
7 (14.9%) |
|
|
Smoker (current or reformed) |
18 (38.3%) |
Table 1 Patient Demographics
|
Characteristic |
Category |
Overall |
|
No. of lesions |
|
N = 96 |
|
No. of lesions (per patient) |
|
1.86 (Mean) (SD 1.36) |
|
|
|
1 (Median) (range 1, 5) |
|
|
|
1, 5 |
|
|
|
n (%) |
|
Lesion Type |
BCC |
25 (26%) |
|
|
SCC |
56 (58.3%) |
|
|
Basosquamous |
1 (1%) |
|
|
KA |
4 (4.2%) |
|
|
IEC |
5 (5.2%) |
|
|
HSK* |
5 (5.2%) |
|
Lesion size |
<2cm |
75 (78.1%) |
|
|
2-5cm |
15 (15.6) |
|
|
>5cm |
6 (6.3%) |
Table 2 Lesion details
*The 10 pre-invasive lesions (HSK, IEC) were all symptomatic.
Treatment
Patients were treated from 2021 to 2023, inclusive. Representative images of the treatment time course (pre-treatment, end of phase 1, end of phase 2, and final follow-up) from two patients were captured (Figure 1). This significantly overlapped the COVID time in Queensland and one reason for performing split course was to decrease the time of these older patients in a health facility to decrease their risk of infection. Up to 5 lesions were treated per patient simultaneously or successively. Most (94.8%; 91 / 96) lesions were treated with electrons, while 3DCRT, IMRT or VMAT were used to treat the remaining 7.3% (7 / 96), all SCCs, due to the curvature of the skin and improved dose homogeneity. There were only 91 treated fields, as some had multiple lesions treated within the same field. The median field area was 19.6 cm2 (range: 7.1, 160) Standard shape electron library inserts of 3 – 10.5 cm diameter, or custom shaped electron inserts were created to treat irregular sized fields.
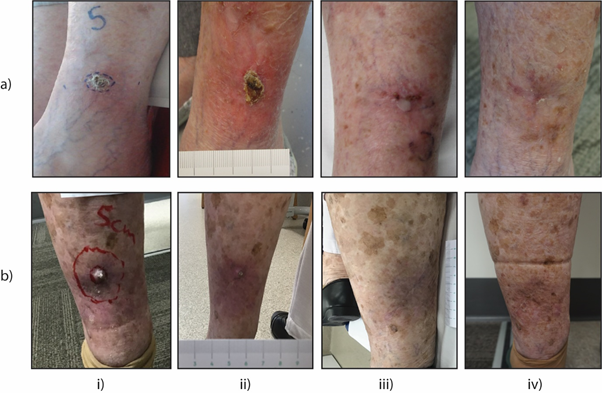
Figure 1 Clinical progression of keratinocyte cancer of the lower limb treated with the ASCRT protocol.
Two representative lower limb cases are presented. a) A 76 - year-old female with a BCC on the left lateral limb and b) a 92 - year-old female with a KA on the left posterior limb were treated with the ASCRT protocol. Images captured at (i) Pre-treatment, (ii) End of Phase 1 (25 Gy), (iii) End of Phase 2 (20 Gy), and (iv) Final Follow-up to demonstrate treatment reactions and disease resolution.
All patients were administered the initial ASCRT phase of 25 Gy (2.5 Gy / 10 fractions) over 2 - weeks followed by an approximate 8 - week break (median: 59 days; range: 34 – 107). Reasons for variation in break-length were clinic scheduling, patient preference or management of unrelated medical comorbidities. The second phase of 20 Gy (2 Gy / 10 fractions) commenced for 65.6% (63 / 96) of lesions from 36 / 47 patients based on a range of factors including treatment response, first phase tolerability, and comorbidities. No lesion increased in size during the treatment break. Treatment was not completed as prescribed for 8.5% of patients (4 / 47) / 6.2% of lesions (6 / 96). Reasons for non-compliance included unrelated illness or medical comorbidities, carer responsibilities or unwillingness to continue treatment due to prior toxicity.
Follow-up
Fifty-five percent (26 / 47) of patients underwent final post-treatment follow-up for this retrospective analysis in-person, with 32% (15 / 47) undergoing final efficacy and toxicity follow-up by telephone. The large number of telephone follow-up reflects the aging population studied with multiple comorbidities. Final cosmetic assessment was only conducted for patients able to attend for in-person review as part of this retrospective study. The median time post treatment to final follow-up of lesions was 16 - months (range 2-30.5 months). Six patients (6 / 47) accounting for 10.4% (10 / 96) of lesions were lost to the final follow-up conducted specifically for this study. The median time to final follow-up of these lesions was 5 months (range: 2 - 11 months). Two patients had died. Efficacy and toxicity assessments for the 6 patients were taken from medical records of routine post-treatment protocols.
Efficacy
Combined treatment efficacy for lesions receiving either 1 or 2 phases (25 Gy or 45 Gy) of ASCRT was 88.5% (85 / 96) CR, 8.3% (8 / 96) PD, and 3.1% (3 / 96) PR according to RECIST criteria (Table 3). All 8 lesions with PD had recurred after an initial CR. Two lesions (2 / 3) with PR received only a single phase of treatment. Two patients (2 / 47) developed a new type of lesion in the treated area within the follow-up period. The median time to recurrence or new disease was 13 months (range 3 - 26 months).
Analysis of efficacy for lesions that completed both phases of the ASCRT protocol (45 Gy total in 20 fractions) yielded CR and PR rates of 98.4% (62 / 63) and 1.6% (1 / 63), respectively (Table 3). One patient (1 / 36) from this group developed a different KC lesion (BCC) in the field following a CR of their previously treated SCC.
|
Cohort |
Response |
Number |
|
|
|
N=96 |
|
|
|
n (%) |
|
Efficacy (whole cohort) |
Complete response |
85 (88.5%) |
|
|
Partial response |
8 (8.3%) |
|
|
Progressive disease |
3 (3.1%) |
|
|
Stable disease |
0 (0%) |
|
|
|
N=63 |
|
|
|
n (%) |
|
Efficacy (full course lesions only) |
Complete response |
62 (98.4%) |
|
|
Partial response |
1 (1.6%) |
|
|
Progressive disease |
0 (0%) |
|
|
Stable disease |
0 (0%) |
Table 3 Efficacy
Treatment efficacy for lesions that were administered a single phase of the ASCRT protocol (25 Gy total in 10 fractions) was 84.8% (28 / 33) CR, 9.1% (3 / 33) PD and 6.1% (2 / 33) PR. The 3 recurrences in this cohort were from 2 SCC and 1 BCC at 3, 12, and 26 months, respectively. The 2 PR were SCC lesions that never fully resolved after 1 phase of treatment.
Salvage strategies for the 13 recurrent, persistent, or new lesions included excision with/without skin graft (5 / 13), RT (5 / 13), all resulting in successful resolution. All skin grafts of the post-RT site were successful. No patients who received salvage RT subsequently developed a radiation induced ulcer or other complications. The remaining 3 lesions continued to be monitored at latest follow-up, owing to patient frailty.
Toxicity
Toxicity assessments were performed by nursing staff and the treating clinician using the CTCAE v 5.0 criteria. Patients commonly exhibited mild to moderate (grade 1 – 2) toxicity (dermatitis, pain, pruritis) during or following the completion of the first phase of treatment. One patient developed a grade 3 ulcer. Patients with larger fungating lesions exhibited more pronounced skin reactions due to tumour breakdown during treatment. Radiation induced ulcers developed in 6.6% (6 / 91) of fields following treatment with a median time to onset of 2.5 months (range: 0 – 12 months). Five patients had completed the split-course regimen. Field sizes tended to be larger where ulcers developed (median diameter: 5.5 cm; range 3 - 8 cm), with 4 developing on the distal leg, foot, or shin that tend to have greater difficulty in healing. Only 1 ulcer developed in a field receiving a single phase of ASCRT (3%; 1 / 33). Two patients who developed ulcers had previously diagnosed vascular comorbidities (insufficiency, T2DM). All ulcers were managed with debridement and dressings, while 1 patient additionally received a skin graft after limited improvement over 6 - months of conservative management. Five ulcers persisted at final follow-up, with a median duration of 3 months (range: 1 - 10.5 months). Of 91 treatment fields, 16.5% (15 / 91) exhibited at least one residual toxicity at final follow-up (Table 4), including pruritis, pain, induration, and ulceration. Two patients reported grade 2 pain, whilst all other toxicities were grade 1 (Table 4). Both patients with grade 2 toxicity related to ongoing ulcer management.
|
Toxicity |
Grade |
Overall (n=91) |
|
|
|
Number (%) |
|
Any event |
1 |
12 (13.2%) |
|
|
2 |
3 (3.3%) |
|
Oozing/Crusting |
1 |
3 (3.3%) |
|
Tightness |
1 |
2 (2.2%) |
|
Pruritus |
1 |
3 (3.3%) |
|
Pain |
1 |
4 (4.4%) |
|
|
2 |
2 (2.2%) |
|
Ulcer |
2 |
5 (5.5%) |
Table 4 Toxicity in fields at final follow up
Cosmesis
From 26 patients (52 lesions) who attended in-person final review, 82.7% (43 / 52) and 7.7% (4 / 52) lesions had excellent or good cosmesis, respectively. Fair cosmesis was recorded for 5.8% (3 / 52) of lesions, owing to pigmentation, telangiectasia, or fibrosis, whilst 3.8% (2 / 52) of lesions had poor cosmesis due to persistent ulceration.
Patient satisfaction
Of 41 patients available for final follow-up, 8 5% (35 / 41) expressed satisfaction with treatment and would receive RT again, whereas 7.5% (3 / 41) were unsure, and 7.5% (3 /41) declined. Patients cited treatment time, travel distance, and ulcers as reasons for responding negatively to future RT.
This study of 47 elderly patients (median 83 years old) with 96 KC or symptomatic pre-cancerous lesions is the largest retrospective analysis of efficacy, toxicity, and cosmesis outcomes for the definitive ASCRT protocol. The 16 - month CR rate of 88.5% for the whole cohort, and 98.4% for the 63 lesions receiving the full ASCRT treatment, was encouraging considering the low incidence of severe toxicity. The CR rate for the full ASCRT protocol aligns with the reported efficacy of conventional RT protocols used to treat lower limbs BCC and SCC.18, 23 These results confirm the malleability of a ASCRT approach to manage complex lesions in high-risk patients and/or anatomically challenging areas like the lower limbs.
The observed incidence of treatment-induced ulcers (6.6%) aligns with, or is superior to, rates for conventional RT protocols across all anatomic sites of 6.3%,24, and 9.2%18 to 14%16 on lower limbs. This is noteworthy given previous studies have tended to exclude patients with peripheral vascular disease and suggests the ASCRT approach does indeed reduce the risk of healing complications. The favourable cosmesis in 90.3% of fields with excellent or good grading is also relevant to overall outcomes, and likely contributed to the high rate of patient satisfaction and overall well-being of 85%.
The high CR rate for lesions receiving both phases of treatment, despite a protracted treatment break, provides valuable evidence towards changing the paradigm for treatment of KC using RT for certain patients. Encouragingly, the protracted break and relatively low dose does not appear to encourage accelerated cancer re-population and/or impact the efficacy of the second phase of RT, common to many other cancers.25 The ability to incorporate a treatment break into KC RT protocols has been described previously,26 although the radiobiology behind the potentially increased sensitivity of KC in the elderly warrants further investigation.19 This study indicates it is possible to further extend treatment breaks to assess initial efficacy or toxicity without compromising overall outcome, thereby personalising the split-course protocol to each patient.
While the extended treatment break in the ASCRT protocol appears to reduce toxicity, defining the parameters of the optimal duration will require further study. The development of ulcers in some patients in this study, particularly those with larger field sizes or existing vascular issues, highlights that this approach does not completely prevent toxicity. Tailored supportive care strategies continue to be important for high-risk patients and this approach may necessitate modification of off-treatment nursing protocols for patients during their break. Encouragingly, the results indicate that a protracted break did not reduce compliance for a second phase of RT if required, with only 4 patients opting out due to comorbidities, social issues, or a toxicity.
With a median follow up of 16 months, this study revealed that the modified ASCRT / Fogarty protocol yielded a high efficacy rate, with a favourable toxicity profile and patient satisfaction rate in this cohort. This supports the foundational principle of the ASCRT protocol, to deliver a safe and effective treatment for patients for whom surgery and / or standard RT protocols are unsuitable.
Future research to build on this approach should focus on larger, prospective studies of RT for lower limb KC using hypofractionated regimens to further reduce treatment burden, and/or the inclusion of other anatomic sites. In addition, formal QoL and health economic analyses of such protocol modifications with respect to fractions, time and treatment burden will also be informative. ASCRT also lends itself to good radiobiological experimentation. It is possible to biopsy the lesions prior to any therapy and then re-biopsy halfway through the course to investigate what is going on at a cellular level.
In conclusion, this study contributes valuable insights into the use of ASCRT for treating lower limb KC or symptomatic pre-cancerous lesions, demonstrating high efficacy and an acceptable safety profile. As ASCRT continues to be refined, it holds promise as a treatment for high-risk patient populations but also as a model for developing more patient-centric RT protocols across different KC presentations.
Authors declare that there is no conflict of interest.
None.

©2024 Wong, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.