International Journal of
eISSN: 2574-8084


Review Article Volume 4 Issue 5
1Department of Radiology, University of Clementino Fraga Filho Hospital, Brazil
2Department of Nuclear Medicine, University of Clementino Fraga Filho Hospital, Brazil
3Department of Nuclear Engineering, University of Federal Rio de Janeiro (UFRJ), Brazil
4Department of Physiotherapym, University of Clementino Fraga Filho Hospital, Brazil
5University of Est
6Department of Medical Physics, Brazilian Nuclear Energy Commission, Brazil
Correspondence: Susie Medeiros Oliveira Ramos, Federal University of Rio de Janeiro, Clementino Fraga Filho University Hospital, Radiology Department, Rua Professor Rodolpho Rocco, 255, Ilha do Fundão Zip Code: 21941-913, Rio de Janeiro RJ Brazil, Tel 55 21 3938-6274, Fax 55 21 3938-6274
Received: November 01, 2017 | Published: November 22, 2017
Citation: Ramos SMO, Thomas S, Pinheiro MA, et al. Internal radiation dose and modeling codes in nuclear medicine: a fresh look at old problems. Int J Radiol Radiat Ther. 2017;4(5):439-443. DOI: 10.15406/ijrrt.2017.04.00111
In the Medical Internal Radiation Dosimetry (MIRD) method, the doses absorbed in the target organs are estimated as a function of activities accumulated in a source organ. Internal dosimetry codes help standardize dose calculations using recognized models, phantoms, and dosimetry techniques. In nuclear medicine community, the aim is to perform calculations to obtain dose estimates for various organs of the body once the kinetics of a radiopharmaceutical agent is established. Accurate dosimetry for each investigation is needed to optimize the use of various alternative nuclear medicine techniques and to estimate the radiation exposure and risk from each procedure. This paper reviews some basic concepts of internal dosimetry codes, key features and difficulties of existing classes for internal radiation dosimetry and modeling codes in nuclear medicine, and the phantoms associated with them.
Keywords: mird method, internal dosimetry, phantoms, nuclear medicine
MIRD, medical internal radiation dosimetry; DT, absorbed dose to a target region; CTL, computed tomography; SPECT/CT, single photon emmission tomography/computed tomography; PET/CT, positron emmission tomography/computed tomography; 3D MRI, tridimensional magnetic resonance; NURBS, non-uniform rational b-spline surface; SI, international System; MC, monte carlo simulation; FDA, food and drug administration; ICRP, international commission on radiological proteCtion; TLD, thermoluminescent dosimetry technique
Absorbed doses of different organs can be estimated by different methods such as Medical Internal Radiation Dosimetry (MIRD). In this method, the doses absorbed in the target organs are estimated as a function of activities accumulated in a source organ and it provides a corrected mathematical estimated dose.1,2 Internal dosimetry codes were designed primarily to lighten manual dose calculations, frequently using tables from MIRD Radionuclide Data and Decay Schemes,3 and ICRP Publication 38.4
With these codes, basic dose calculations could be made in seconds instead of hours. Another purpose of these codes was to help standardize dose calculations using recognized models, phantoms, and dosimetry techniques. In nuclear medicine community, the aim is to perform calculations to obtain dose estimates for various organs of the body once the kinetics of a radiopharmaceutical agent is established. The administration of radiopharmaceuticals to humans for diagnostic, therapy or research purposes is a well-established and developing branch of the nuclear medicine practice. New methods and radiotracers are continually introduced. Accurate dosimetry for each investigation is needed to optimize the use of various alternative nuclear medicine techniques and to estimate the radiation exposure and risk from each procedure.5
Many research groups that work in internal dosimetry have been developing their own tools, and day by day new software is being published.6 This paper reviews some basic concepts of internal dosimetry codes, key features and difficulties of existing classes for internal radiation dosimetry and modeling codes in nuclear medicine, and the phantoms associated with them.
Basic Concepts
To understand the calculations performed to obtain the total absorbed dose (DT) to a target region through a certain code, firstly the user needs to know basic internal dosimetry concepts, such as target and source regions, S values, and cumulated activity in each organ. Herewith the main definitions are as follows:
Source region
Region that holds a radiopharmaceutical for a specific amount of time related to the biological half-life of the pharmaceutical, and it is emitting ionizing radiation to other regions nearby.7
Target region
Region that is exposed to the ionizing radiation from the source regions.7
Cumulated activity
The level of activity obtained at different times after injection, plotted against time, gives a time-activity curve for a particular organ. The integral of this curve gives the total number of disintegrations or the cumulated activity for the region, which must be determined for each patient.7
S value
The absorbed dose to a target region per unit cumulated activity in the source regions.7
Absorbed dose
Expressed in units of Gray (“rad” in older texts; 1Gy=100 rad), it is defined as the energy absorbed in a particular mass of tissue, divided by the tissue mass. In internal dosimetry, DT is the goal of the calculations, and it is defined as the sum of mean dose contributions to the target from different source regions.7
Stylized, voxel, hybrid and physical phantoms – why do they exist?
It is well known that a precise representation of the human body is important for accurate internal dosimetry. The ideal situation would be to use the diagnostic image of the patient as anatomic model to estimate doses received by the diagnostic image itself or by a previous nuclear medicine treatment. Such “patient-specific” anatomic models can and are used in a research-level, where the Computed Tomography (CT) portion of Single Photon Emmission Tomography/Computed Tomography) SPECT/CT or Positron Emmission Tomography/Computed Tomography (PET/CT), or even Tridimensional Magnetic Resonance (3D MRI) as shown by Berdeguez et al.8 could be used for radiation transport simulation and new dose planning methodologies.8 Problems of image-based patient-specific models include the lack of whole-body coverage in most cases and the need to segment those images to quantify organ absorbed doses separately.
Anthropomorphic phantoms exist to simulate the human body’s reaction to ionizing radiation, and allow whole-body or partial dosimetry in the organ of interest. The stylized phantoms have the size and form of the body and its organs are described by mathematical expressions (combinations/intersections of planes, circular and elliptical cylinders, spheres, cones, etc.).9 Voxel phantoms are based on digital images recorded from CT or MRI.10 Hybrid phantoms are the combination of both, stylized and voxel phantoms. The hybrid approach to a computational phantom construction is based on non-uniform rational B-spline (NURBS) surface animation technology that has the advantages of both former phantom generations.11 These three generations are shown in Figure 1.
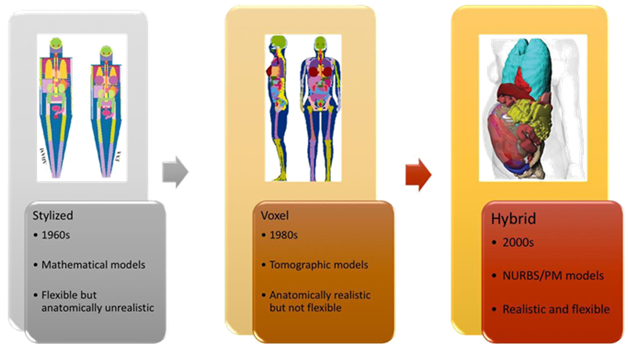
Figure 1 From left to right: examples and characteristics of the first [13], the second [14] and the third [15] generation of computational phantoms.
Both computational and physical phantoms are progressing, since their response can only be validated with one another. When it comes to radiation protection of the patients, new phantoms are not only providing data for new and improved internal dosimetry software, but also for dose optimization studies, dose planning, and research on the biological effects of ionizing radiation.12
Internal dosimetry codes
MIR dose: MIRDose was developed and distributed by the Radiation Internal Dose Information Center, Oak Ridge Institute for Science and Education, USA. The software contains tables of the S values for common radionuclides. The user must provide the biokinetic data in residence times for each source organs. It then generates tables of organ doses per unit administered activity in rad/mCi, and in International System (SI) units mGy/MBq.16,17
Versions 2 and 3 used pediatric mathematical phantom series for the photon-specific absorbed fraction libraries, giving the user the ability to calculate dose estimates for adults (70kg), 15-yr-olds (57kg), 10-yr-olds (32kg), 5-yr-olds (19kg), 1-yr-olds (9.2kg) and newborns (3.4kg). These models were based on the 1974 adult phantom of Snyder et al.18 This adaptation is exemplified in Figure 2. The 15 age phantom was modified to represent both a 15year-old male and an adult female. This was justified by the body weight and dimensions of a reference adult female from ICRP 1975,12 which was close to those of the 15yr-old.19,20 Figure 2 also shows the active bone marrow considered in the mathematical model from 1974 and the one considered in MIR dose software.20

Figure 2 Mathematical phantoms from Snyder in 1974 - (A) and the one improved for MIRDose calculations (B). The shaded areas are the active bone marrow [20].
Main features: The software has first-generation phantom libraries which allow the calculation of absorbed organ doses for individuals of different ages and sizes, and for women at different stages of pregnancy.1
Difficulties: The user must describe the biokinetics of the radiopharmaceutical1 (from animal or human data) or find them in the literature. However, radiopharmaceuticals’ kinetic models are constantly being updated as new data becomes available to improve them. Also, it is still questionable whether the model for the 15yr-old should be used as an adult female.
Currently, MIR Dose 3.0 and 3.1 codes are still being used for absorbed organ dose calculations. For instance, it is used to address a therapeutic dose in an organ after a tracer patient-specific dosimetry.21 Also for absorbed dose determination in non-target organs22 and for development of new radiopharmaceuticals.23
MABDOSE
MABDOSE was developed at the University of Colorado. It is another package based on fixed geometries.
Main features: It allows the user to place spherically shaped tumors within the simplified anatomic model originally described by MIRD Committee. Monte Carlo (MC) simulation was incorporated into this code. This method accommodates tumor dosimetry within the idealized geometry defined by the MIRD Committee.24,25 Unlike MIRDose 3, it can estimate the dose to adjacent organs as a result of the tumor activity and the dose to the tumor as a result of activity in adjacent tissues by calculating S values for metastases as source or target organs, treating the tumor as a sphere within the Reference Man geometry and performing MC simulation in real time.26
Difficulties: It uses fixed geometries from the first-generation mathematical phantoms, hence it cannot be used for patient-specific dosimetry.
OLINDA/EXM
The code OLINDA/EXM, Organ Level INternal Dose Assessment/EXponential Modeling, was designed as an update to MIR Dose.
Main features: The nuclear medicine dose factors were published by the same developers of MIRDose, but for many more nuclides (over 1000 vs 117), including some alpha emitters.27 It uses the second-generation voxel-based realistic phantoms for adults, children and pregnant women, based on ICRP 89 reference organ masses, besides animal models for mice, rats, and dogs. It can modify organ masses to patient-specific values and assign activity to the walls of hollow organs.28 In the EXM code portion, users can perform kinetic analyses, fitting sums of exponentials to data gathered in animal or human studies.28
Difficulties: Version 1 was FDA approved. However it was withdrawn from the market and replaced by Version 2, which is the only one currently available, and users also have to enter biokinetic data into the code.
Tridimensional (3D) dosimetry codes
3D patient-specific dosimetry better accounts for radionuclide distribution and anatomic patient variability. Cumulated activity data need to be input in these codes, which are usually obtained from SPECT and nuclear medicine planar imaging studies.29
DOSIMG
This software has been used with mathematical phantoms to analyze different quantitative SPECT algorithms on MC-derived absorbed dose calculations.
Main features: Mathematical phantoms are generated through CT and SPECT images and dose calculations derived from these are compared with “true” dose results derived from the actual mathematical phantom data.30 The methodology can be applied to 111In/90Y dosimetry.31
Difficulties: some inaccuracies were imposed by the SPECT system limited spatial resolution, for which the collimator response correction did not fully compensate. Compensation methods are necessary, based on physical models.
Royal marsden dosimetry package–RMDP
MIRD Committee has also published S value tabulations for different voxel sizes and source-target voxel distances.32 The resulting S value tabulations reduce the lengthy and complex MC calculations to estimate absorbed dose. Since previously tabulated S values used a fixed anatomic model, geometries deviate significantly from one another.33 Voxel S values were developed to overcome this problem and have been adopted in several dose calculation programs, such as RMDP, a dedicated package for Iodine-131 (131I) therapy.34,35
Main features: it includes full photon interactions in a hexagonal-hole collimator and the gamma camera crystal. This code has led to improved 131I image quantification and contributes towards 3D dosimetry.36
Difficulties: Its accuracy is limited by the quality of the cumulated activity data that is entered in the code, which is the same difficulty found for most codes.
Voxel dose
This code was developed to calculate patient-specific 3D-dose maps at the voxel level. This software was validated with a physical abdominal phantom, the Liqui-Phil™, with four organ inserts and one spherical tumor filled with a known activity concentrations of Indium-111 (111In), combined with the Thermoluminescent Dosimetry technique (TLD).37
Main features: The SPECT acquisitions are reconstructed using a filtered back projection method, with attenuation and scatter correction; the 3D cumulated activity map is generated by integrating SPECT data; and a 3D dose map is computed by convolution using the Fourier Transform of the cumulated activity map and corresponding MIRD voxel S values.37
Difficulties: It could not precisely associate the position of the TLD to a voxel and it had limits of the quantification method, such as scatter correction and partial volume effect.37
3D-ID
It is an ongoing-investigation code in 3D internal Dosimetry used for research. Multiple planar images are used combined with a single SPECT image. The planar images are used to obtain kinetic information, and SPECT images for better spatial distribution of radioactivity.29 This software introduced the concept of dose-volume histograms for internally administered radionuclides.38 It may be used to perform both Monte Carlo and point-kernel-based calculations, and to examine the impact of different radionuclides on dose distribution, given a fixed cumulated activity distribution.29
Main features: it was able to perform a dosimetric analysis of 131I-labeled anti-CD20 antibody therapy for patients with non-Hodgkin’s lymphoma. It has also been showing that it is feasible to perform Iodine-124 (124I) PET–based patient-specific 3D dosimetry, and that sequential PET can be used to obtain cumulated activity images for 3D dosimetry.39
Difficulties: To obtain 3D cumulated activity images, the registered image sets need to be integrated, voxel by voxel, over time. Isodose contours need to be drawn over the images to provide information regarding absorbed dose distribution within the tumor volume.39
RADAR
It is a code that use anatomical models based on NURBS hybrid models to determine absorbed dose in tissues and organs.40
JADA
It is a MATLAB graphic interface that can be used to process data obtained by planar imaging or SPECT. Activity data in each time for source regions can be obtained by a set of tools. This software uses the same OLINDA phantoms and absorbed doses are also estimated by MC simulations.41
CELLDOSE
It is a C Language Code used to estimate absorbed dose from low-energy-electron irradiation (from 10eV to 1 MeV) on a cell level.42
DoRadIo
The Ionizing Radiation Dosimetry (DoRadIo) is a code that receives information from source organs and target organs and generates graphical and numerical results. It was developed by MASH voxel simulation combined with EGSnrc MC code. Radiopharmaceutical biodistribution and kinetics are estimated through imaging and pixel intensity variation in a region of interest.43
Some codes have been created to estimate occupational radionuclides ingestion or inhalation, based on the International Commission on Radiological Protection (ICRP) published dose coefficients.
Activity and internal dose estimates-AIDE
This code is used for both in vivo biodistributions and in vitro assay data studies. Its main application is in radiation safety and estimates of absorbed doses associated with occupational ingestion of radionuclides.44
Program for linear internal age-dependent doses-PLEIADES
Developed by the UK´s Health Protection Agency, aims to solve the problems related to biokinetic.45
It is well-known that MIRD provides an average equivalent dose in the target organ and do not address the maximum absorbed dose. Spatial dose distribution can be achieved by voxels model simulation discriminating absorbed dose in a specific part of the organ such as an artery, for example. Such improvement can contribute to better specify deterministic effects of radiation in the organs. As nuclear medicine expands to include new therapeutic and diagnostic procedures, as well as new radiopharmaceuticals, the importance of radionuclide dosimetry has also been increasingly important. Radionuclide dosimetry should be used to plan radionuclide therapy in the same way that dosimetry is used to plan external beam radiotherapy. It is important to understand and distinguish the response between biological and dosimetry factors, to achieve treatment planning in nuclear medicine therapies. Therefore, it is essential to nuclear medicine community that ongoing improvements in dosimetry methodologies and codes continue so that the role of dosimetry, relative to biological factors could be properly evaluated.
We would like to thank the CAPES and CNPq programs for granting scholarships that allow us to continually work for the progress of science and knowledge for our country.
Author declares that there is no conflict of interest.

©2017 Ramos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.