International Journal of
eISSN: 2574-8084


Review Article Volume 2 Issue 4
1Radiology Department, Federal University of Rio de Janeiro, Brazil
2Nuclear Medicine Department, Clementino Fraga Filho University Hospital, Brazil
3Nuclear Engineering Department, Federal University of Rio de Janeiro (UFRJ), Brazil
4Medical Physics Department, Brazilian Nuclear Energy Commission, Brazil
Correspondence: Susie Medeiros Oliveira Ramos, Federal University of Rio de Janeiro, Clementino Fraga Filho University Hospital, Radiology Department, Rua Professor Rodolpho Rocco, 255, Ilha do Fundão Zip Code: 21941-913, Rio de Janeiro RJ Brazil, Tel 55 21 3938-6274, Fax 55 21 3938-6274
Received: January 24, 2017 | Published: March 14, 2017
Citation: Ramos SMO, Thomas S, Berdeguez MBT, et al. Anthropomorphic phantoms-potential for more studies and training in radiology. Int J Radiol Radiat Ther. 2017;2(4):101-104. DOI: 10.15406/ijrrt.2017.02.00033
Anthropomorphic phantoms are used for the assessment of image quality and to simulate a medical procedure. They can be likewise developed for training and teaching for all modalities of imaging. Phantoms built from tissue-equivalent materials provide a physical representation of the anatomy of the human body and attenuation characteristics, allowing researchers to calculate absorbed organ doses, improving treatments effectiveness and protecting healthy tissues. Now a days, physical phantoms have been used as a comparison to computational models for validation of Monte Carlo codes, which are computational phantoms. The more complex the tissue is structured, the more difficult it gets to design the phantom. With the development of 3D printers, new phantoms can be built from medical imaging. However, current 3D printers do not use a wide variety of materials, so it is not possible yet to create phantoms for functional imaging. Studies must be performed to optimize current imaging techniques and elevate their accuracy, which would provide visual, practical, hands-on training of the medical staff, with real-world simulations, ideally patient-specific. This technological advance should be made with physical and computational phantoms in parallel since validation has a high value to the reliability of this scientific method. When it comes to radiation protection of patients and staff, new anthropomorphic phantoms could also provide data for new dosimetry and radiation protection studies, serving as a foundation for the progress of radiological safety throughout the world.
Keywords: anthropomorphic phantoms, physical phantoms, computational phantoms, patient-specific simulations, radiological, radiation protection studies, dosimetric, soft-tissue, cautionary reports, energies, quality control, sophisticated
CT, computed tomography; BMI, body mass index
An educational update on anthropomorphic phantoms
Since 1906, there have been researches on tissue substitute materials to study the dosimetric effects within and around irradiated tissues. From that moment on, phantoms in one form or another have been used extensively in experimental radiation dosimetry.1,2
During the 1920’s, water and wax were established as muscle and soft-tissue substitutes.1‒3 A decade later it was reported that the attenuation coefficients of wax at low photon energies differed widely from those of muscle and soft tissues.4 Phantoms produced from the improved wax-based tissue substitutes with high atomic number fillers, in particular, Mix D 5 and M3,6 were employed widely in radiation dosimetry.1 Dosimetric wood-based phantoms were used for manyyears,7‒10 but cautionary reports have been made about the variable photon attenuation properties of wood.11,12 A few papers on realistic phantoms could be found in the literature13‒15 since most researchers at that time appeared to prefer phantoms with simpler geometries.1
The greater awareness of the importance of realism in phantom body design was illustrated back in the 1940’s and 50’s.16‒20 In the early 60’s, the trend towards innovative body phantom design continued with the introduction of two elaborate adult-sized body phantoms, the ‘Temex’21 and the ‘Rando’.22 They had real skeletons, body cavities, and artificial lungs, with slices that enabled the evaluation of radiation dose-distributions.
From 1970 to the present day, more phantoms have been produced for applications besides radiotherapy dosimetry, such as radiodiagnosis and radiation protection in medical imaging,23‒26 and for evaluating the performance of all imaging techniques, like CT, by the American Association of Physicists in Medicine.27 Figure 1 illustrates the improvements made in phantoms design from 1970 to nowadays.
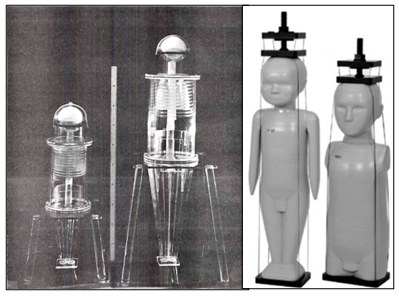
Figure 1 Anthropomorphic phantoms of one and five-year-old children in 1970 (left) and 2015 (right) [29,30].
In 1982, White and Constantinou published the book “Progress in Medical Radiation Physics”,28 in which they stated that measuring equipment and clinical techniques had become more sophisticated, so the types of tissues needing simulation and the degree of realism required between replicating materials and real tissues needed to be increased. Radiation physicists were then no longer satisfied to use simple blocks of solid materials or tanks of liquids but demanded composite arrays of substances and phantoms having ever-increasing degrees of realism. It has been thirty-five years since that statement.29,30 So, are we there yet? Have we evolved that much?
Phantoms for quality control, anthropomorphic phantoms, and computational phantoms
Quality control phantoms are used for the proper operation of imaging equipment, supporting in the inspection of parameters such as spatial resolution, energetic resolution, linearity and the center of rotation, intrinsic and extrinsic uniformity, among others.31
Anthropomorphic physical phantoms are the ones in the shape of a human body or part of it, manufactured with materials that are equivalent to human tissues when it comes to size, shape, positioning, density and radiation interaction with matter.32 They can be used in radiotherapy, nuclear medicine, radioprotection studies and radiobiology.33 Figure 2 illustrates examples of anthropomorphic physical phantoms and quality control phantoms. Currently, anthropomorphic physical phantoms have also been used for experimental studies to validate computer simulations by comparing directly both dosimetric data.35‒40
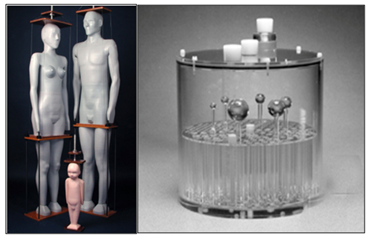
Figure 2 Anthropomorphic physical phantoms (left) and a quality control phantom model Deluxe Jaszczak Phantom from Data Spectrum Corp (right) [31, 34].
The benefit from modeling a computational human phantom is on its cost of production and necessary infrastructure, aside from enabling greater flexibility on simulations, as demonstrated in Figure 3. The RPI model allows an adjustment of the BMI of the phantom according to the medical condition of the patient, from normal weight to overweight and morbid obesity.41
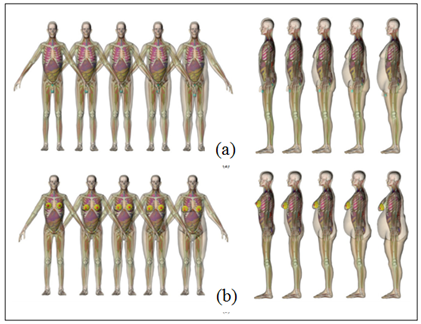
Figure 3 Anterior (left) and lateral (right) positioning of a man (a) and a woman (b) from ultra-realistic 3-D computer models, the RPI computer models [41].
In a similar way as physical phantoms, computer models have gone through some significant evolution in their geometric sophistication,42 as illustrated in Figure 4. They are improving alongside with the evolution of physical phantoms, which are required for the validation of data obtained by the computer simulations.
The more complex is the tissue or organ structure, the more complex becomes its physical or computational phantom development.43 For instance, in the case of joints, there are no phantoms that could be used for the analysis of their entire anatomy structure. A knee joint phantom should include skin, muscles and ligaments, bones, articular cartilage, synovial membrane, synovial liquid, fibrous capsule, among others, all built with tissue-equivalent materials. This degree of realism would provide more accurate data in dosimetric studies of conditions such as chronic synovitis - occasionally treated with radiosynovectomy - also valuable for visual medical training.30,34, 44,45
With the advancement of 3D technologies, new phantoms have been built using 3D medical imaging, for instance:
The printers commercially available nowadays do not have a wide variety of materials (with different densities and compositions). Therefore it is only possible to build anatomical phantoms for structural images, non-functional, which does not reveal physiological activities on the tissue or organ.46‒48 With the limited choices of available plastics to use on 3D printers, it could be employed Hounsfield Units to select the closest material as an equivalent tissue one.47
Recent studies have shown the need for patient-specific phantoms since current physical phantoms rarely represent with accuracy one specific patient or a cohort of interest.48 The physiology of different groups according to age, gender and BMI have been studied during the development of new phantoms. However, there is still some considerable variety among the organisms.48‒50
There is a substantial need for optimization in nowadays protocols when it comes to patient-specific dosimetry.47‒50 Current studies48 have shown the viability and benefits of patient-specific simulations. Oncology and Radiation Therapy are medical fields that could benefit the most with this technology47,49,50 since the geometry of the tumors is significantly variable, with substantial effects on dose planning and quantification accuracy.
A study in 201448 investigated the possibility of using 3D printers as a rapid prototyping technology for the production of patient-specific, cost-effective phantoms directly from patient CT data. The study concluded that 3D printed phantoms based on CT data show good correspondence with anatomical references (structural phantoms), although there is still a lack of phantoms that also represent physiological activities of the organs or tissues (functional phantoms). The methodology developed can be applied to other anatomical or geometrical phantoms for molecular imaging.
The advancement of this technology requires contributions from the entire scientific society to develop more realistic, flexible, cost-effective phantoms to be used in the medical routine for diagnostic imaging and treatment planning.
Why would the world need more realistic physical phantoms, both structural and functional? Because there is still room for improvement in current imaging techniques, which requires optimization when it comes to radioprotection, time and cost for each procedure, and the methodology itself. The aim is to obtain more efficient treatment, with the right radiation dose to the target organ, besides protecting healthy tissues while preserving their function.
New realistic phantoms could also provide visual, hands-on training of the medical staff, with simulations so close to reality that, ideally, would represent different individuals and would be patient-specific. This advancement must occur for both physical and computational phantoms since their response can only be validated with one another.
When it comes to radiation protection of the patients and the staff, new phantoms could also provide data for calculation of doses and the biological effects of ionizing radiation, serving as a foundation for the progress of radiological safety throughout the world.
None.
Author declares that there is no conflict of interest.

©2017 Ramos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.