International Journal of
eISSN: 2574-8084


Educational Review Volume 9 Issue 4
1Icon Cancer Centre, Revesby, New South Wales 2122, Australia
2Icon Cancer Centre Wahroonga, Sydney Adventist Hospital, Wahroonga, New South Wales 2075, Australia
3Strategic Investment and Clinical Care, Icon Cancer Centre, Queensland 4101, Australia
Correspondence: Gerald B. Fogarty, Icon Cancer Centre, Revesby, New South Wales 2122, Australia, Tel +61 (2) 87222800, Fax +61 (2) 87222801
Received: August 09, 2022 | Published: August 26, 2022
Citation: Fogarty GB, Cooper B, Hodson MA, et al. A scientific approach to skin radiotherapy nursing Article 4 – How nurses can predict, explain, and care for the acute side effects that may arise during a course of skin radiotherapy. Int J Radiol Radiat Ther. 2022;9(4):105-114. DOI: 10.15406/ijrrt.2022.09.00332
Nurses play an important role in the care of patients treated with radiotherapy (RT) for skin conditions, such as skin cancers. This is the final article in a four-part series that aims to supplement general oncology nurse training by providing a scientific basis to better understand a) the use of RT in skin and its diseases; and b) the management of acute skin RT reactions to achieve best patient outcomes.
The first article focused on the role of the nurse within the radiation oncology department and the initial patient assessment. The second article revised the anatomy, physiology, pathologies and radiobiology of skin. The third described the skin RT prescription and plan, especially in relation to volume and dose.
The aim of this fourth article is to assist the nurse, by applying the knowledge from the previous articles, to predict, explain and care for the acute side effects that may arise during a course of skin radiotherapy.
Keywords: radiotherapy, radiobiology, superficial radiotherapy, volumetric modulated arc therapy, skin, skin cancer, nursing, review
Nurses play an important role in the care of patients treated with radiotherapy (RT) for skin conditions, such as skin cancers. This article is the fourth is a series of four articles1,2,3 that aims to supplement general oncology nurse training by providing a scientific basis to better understand the skin and its diseases, the use of RT in skin, and the management of necessary acute skin RT reactions to achieve best patient outcomes.
The first article1 focused on the role of the nurse within the radiation oncology department and the initial patient assessment. The second article2 revised the anatomy, physiology, pathologies and radiobiology of skin, and the third3 described the skin RT prescription and plan, especially in relation to volume and dose.
This fourth article aims to assist the nurse, by applying the knowledge from the previous articles, to predict, explain and care for the acute side effects that may arise. A glossary of terms used throughout this paper is outlined in Table 1.
Word |
Meaning |
Accelerated |
A hypothesis that explains the observation that local control rates after RT decrease when overall treatment time increases. |
Arteriosclerosis |
Hardening and narrowing of the arteries. |
Fistula |
Abnormal connection between two body parts, such as an organ or blood vessel and another structure. |
BCC |
Basal cell carcinoma |
“Belling out” |
The wide penumbra of electrons that can increase under the skin. |
cSCC |
Cutaneous squamous cell carcinoma |
CTV |
Clinical target volume |
CT |
Computed tomography (scan) |
CTh |
Chemotherapy |
DVH |
Dose-volume histogram |
EAC |
External auditory canal |
GTV |
Gross tumour volume |
IVD |
In vivo dosimetry |
MV |
Megavoltage treatment |
OARs |
Organs at risk |
PTV |
Planning target volume |
RO |
Radiation oncologist |
RT |
Radiation therapy/radiotherapy |
“Shine through” |
The RT beam continues to penetrate through the body and surfaces on another epithelial surface. |
SM |
Second malignancy |
SXRT |
Superficial radiotherapy |
Table 1 Glossary and explanation of terms used in this paper
Importance of minimising the side effects of RT
Acute effects: Acute side effects of RT are a sterile acute inflammation. RT results in the classic hallmarks of acute inflammation: redness, heat, swelling, pain, and a loss of function in normal tissues within the treatment volume. The aim is to keep the side effects to a minimum and tolerable so that treatment can still proceed and be completed on time. If treatment is paused, delayed or stopped because of acute toxicity, treatment may fail. Radiation nurses are involved in implementing strategies on a day-to-day basis aimed at minimising acute effects to ensure course completion, patient comfort and optimal outcomes.
Radical doses of RT will produce side effects in normal skin and other normal tissues within the high dose treatment volume. Acute side effects arise in a predictable manner, especially in hierarchically organised renewing adult cell populations like skin, as discussed in article two.2 Acute side effects in skin within the high dose volume will peak four weeks after the start of RT in patients receiving a radical dose and daily (five days per week) treatment.2 When treatment equals or exceeds four weeks, and normal in-field skin also receives the full dose, side effects will usually peak at the end of RT. For those receiving a radical dose in less time, side effects will still peak at four weeks. This is observed in the case study outlined in Figure 14 of article two2 in which the patient received 45 Gy in 15 fractions over three weeks. The peak normal skin side effect occurred four to five weeks after the start of RT.
Treatment delay or cessation in malignant disease may lead to rapid tumour regrowth due to a process known as accelerated repopulation that is best documented in mucosal SCC.4 Accelerated repopulation is a process that explains the clinical observation that, in some types of cancers, local control rates after RT decrease when overall treatment time increases. Whether accelerated repopulation happens in skin has not been shown conclusively; however, some guidelines recommend that RT in cutaneous squamous cell carcinoma (cSCC) should not be delayed.5
High quality nursing care is fundamental to timely treatment completion. Overall treatment time can increase with breaks. Unplanned breaks can occur when acute effects become too much for the patient to bear. Keeping these to a minimum with high quality nursing care is essential to a good outcome.
Late effects: Skin disorders treated with RT need a certain total dose which is usually delivered in a number of fractions. As seen in article two,2 fractionation is essential to minimise the late effects of RT. Late effects are defined as starting six months post RT completion. They are dominated by fibrosis and result from the death of normal cells, especially those in hierarchically organised cell populations, through the swamping of the RT repair mechanisms in normal cells due to large fraction sizes.
A classic late effect is fistula formation. A fistula is an abnormal connection between two body parts, such as an organ or blood vessel and another structure. Fistulas caused by radiation are usually formed within volumes of tissue at high risk of infection such as the oral cavity, vagina, perineum, rectum, and anus. Often fistula are also associated with previous instrumentation. Usually, the potential infection would be stopped by blood flow to the affected volume. However, because radiation-induced fibrosis has caused arteriosclerosis to the arteries which supply the volume, the infection can get a foot hold. The infection will continue to destroy tissue until it runs into a volume of tissue that has enough blood supply to stop the advancing infection. In the meantime, an abnormal connection may have been formed, such as a rectovesical fistula - a connection between the rectum and the bladder. This can cause urine and faeces to mix and empty out of the urethra, requiring significant corrective treatment to reverse it.
Another late effect is radiation ulcer formation, described as a consequential late effect in article two.2 Radionecrotic ulcer can also occur following instrumentation within an irradiated field. These potentially painful ulcers happen because of insufficient vascular supply to heal the wound. Surgeons are therefore cautious about operating in an irradiated field. ROs are similarly cautious about delivering RT to an area with known vascular insufficiency.
A further classic late effect is osteoradionecrosis of the mandible. This complication occurs when dental decay is not kept in check by a good blood supply due to radiation. It is possibly caused by vascular insufficiency following arteriosclerosis of the mandibular artery. Dental decay therefore needs to be assessed and managed by a dentist prior to treatment in situations where the mandible will be in the treatment field. Nurses may be asked to help cure late effects by conservative measures until healed, such as continuous dressings.6
Finally, another rare late effect is second malignancy (SM). This occurs in irradiated normal tissue. This is less with modern RT thanks to better dose conformity as described in article three.3 The risk is one in one thousand at ten years. That is, for each one thousand patients irradiated, there will be a radiation-induced cancer arising in one of the one thousand for every decade of life they live.7 This is one reason why some consider sixty to be a reasonable age at which RT can begin to be offered, but there is no hard data on this. There is data to suggest that below this age the risk may be higher,8 but this was with older and less conformal RT. SM risk is more of a consideration for those being treated for benign disease. It is a good example to stress the importance of clear communication about risk and the need for informed consent prior to surgery, particularly for example for a younger person being treated with post operative RT for a keloid. The usual RT induced cancer in skin is a basal cell carcinoma (BCC) which should be treated on its merits.7
Decreasing side effects in RT is the responsibility of all: The responsibility to decrease side effects is a responsibility shared by all RT craft groups.
The best way to avoid RT side effects in surrounding normal tissue is to not irradiate it. The RO needs to know what volumes of normal tissue to include and exclude in the high dose volume, especially in what constitutes the clinical target volume (CTV).9 The RT planners need to create a plan that excludes the presence of normal tissue in the high dose volume as much as possible; that is, with high dose conformity with no detriment to homogeneity. The RT treatment staff need to carefully set up the patient each day so that the exact same volume is treated with every fraction. The physics team’s quality assurance needs to ensure that the prescribed dose is being delivered to the correct volume. The engineering team needs to continue to develop RT machines and planning systems that are precise. Significant progress has been made across all these areas, making the nurse’s job much easier by default.
Understanding the high dose RT volume from the RT prescription and plan: A significant part of the nursing role is to manage the acute effects in normal tissues that cannot be excluded from the treatment volume. To do this well, it is helpful for RT nurses who manage skin patients to know how to predict the volumes that are being irradiated. The nurse can know the volume from the prescription and the plan. However, the irradiated volumes will be different for each different treatment modality, given the differences in penumbra and beam penetration.
Superficial radiotherapy (SXRT): For superficial radiotherapy (SXRT), the volume will be the size of the treatment area, or field on the skin, multiplied by the penetration depth of the beam. The area will be seen in the photos taken by the radiation therapist at planning. Figures 11 and 12 in article three3 provide examples of SXRT fields drawn on noses. The depth will be known by the half value layer (HVL) as also described in article three3. The planning photos allow the nurse to know the anatomical site of SXRT and the direction of the SXRT beam. It is then easy to predict the acute RT effects that will affect infield tissues and surrounds and the associated timeframe. Figure 1 documents a case study where astute nursing helped explain an unexpected acute reaction. This is an example of “shine through”, which is when the SXRT beam continues to penetrate through the body and exits on another epithelial surface.

Figure 1 A case study where astute nursing helped explain an unexpected acute reaction. A middle-aged woman having definitive SXRT for a basal cell carcinoma (BCC) on the left upper nasal alar (black arrow) noticed redness on the right alar (blue arrow) at the halfway point of a prescribed course of SXRT. This redness was in the epidermis, a hierarchically organised cell population. Initially the RT staff thought this reaction was due to how the SXRT cut out was taped to the skin and sought the nurse’s opinion. The nurse thought that this could be due to “shine through” from SXRT. The physics team was consulted, and in vivo dosimetry (IVD) was conducted. Doses high enough to cause redness were measured. “Shine through” is when the SXRT beam continues to penetrate through the body and exits through another epithelial surface. In this case, the air in the nasal vestibules meant that less dose was absorbed in the SXRT path, enabling the skin on the contralateral side to be irradiated. The radiation oncologist (RO) was consulted to consider replanning, but this may have required reorientation of the beam which would have compromised the gross tumour volume (GTV) cover and sent dose into other normal tissues. The patient was counselled and agreed to continue the course, which was completed with no further issues. The area affected by the shine through healed spontaneously after RT completion.
For megavoltage (MV) treatment, the treatment volumes and organs at risk (OARs) are defined by the RO, who usually contours them on the planning computer tomography (CT) scan. The planning radiation therapist then computes the dosimetry according to the prescription and presents this to the RO for approval.
The resultant plan can be displayed graphically on the planning system as shown in the prostate plans in article three3 Figure 2. As the volumes and doses are known, a dose – volume histogram (DVH) can be constructed (Figure 2). The aim of planning is to obtain a homogenous dose to the PTV that fits the volumes requiring treatment with the best conformity, and to spare the OARs from any dose. This usually means a compromise as OARs are often close to the treatment volumes. How much compromise is acceptable is weighed up when the RO approves the plan. In difficult cases, the plan will be discussed amongst peers to ensure there is agreement about the adequacy of PTV coverage for cure and the acceptable dose to OARs. This meeting can have different names in different departments; for example, ‘peer review’ or ‘chart round’. Meeting organisers should ensure that nurses are invited and represented as a workgroup at these meetings as acute side effects can often be predicted based on the plan DVH.
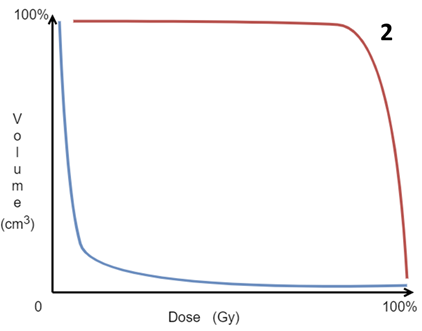
Figure 2 As the volumes of the target and OARs are known, and doses have been computed through the treatment volume, a dose – volume histogram (DVH) can be constructed. Volume is on the vertical Y axis and dose is on the horizontal X axis. The aim of RT is to cover all the volume of the PTV with as much of the prescribed dose as possible, so on the DVH, the PTV line should approximate the red line. The OARs should receive the least dose to the smallest part of the volume, ideally where the blue line is.
The dosimetry can be displayed in many ways, but a useful way is with the dose colour wash option on the planning CT slices. The highest dose volume is depicted in red. Red changes through the colour spectrum to blue, which represents lower doses as the amount of dose decreases away from the target volumes. This is shown in Figure 2 in article three3 with prostate planning.
|
Practice point for predicting acute RT side effects during treatment |
|
As a rule of thumb, to get some idea of what acute side effects may arise during a radical course of megavoltage RT, one can look for organs that have a hierarchically organised renewing adult cell population (e.g., skin, gut, mucosa etc.) that are within the dose cloud of half of the radical dose prescription. These are the organs that may suffer acute side effects during the RT course. > |
For example, if the total dose is 60Gy, the nurse can ask the planners to show the approved plan in dose colour wash mode and look for the volume of normal tissue that will receive 30Gy. Organs within that volume that have a hierarchically organised renewing adult cell population are at risk. This helps the nurse to predict and explain the possible acute effects to the patient. It also serves to set patient expectations and can be used to prepare the patient on what acute effects to look for and how to minimise them. This principle will be demonstrated in the following case studies.
Megavoltage (MV) electrons: Electrons rapidly fall off with depth so side effects in deeper tissues are rare. However, they do suffer from a wide penumbra, as explained in article three,3 and the dose cloud can “bell out” under the skin. Figure 3A documents a case study where astute nursing helped explain an unexpected acute reaction from electrons.

A Planning photo of an elderly man with symptomatic actinic field change anterior to the left pinna that had grown invasive disease. A lesion within the area of concern had been recently excised and found to have a positive deep margin on histopathology. The solid line indicates the CTV. The dotted line indicates the planned electron field.

B Planning axial computed tomography (CT) scan through the volume showing the contour of the planning target volume (PTV) in light blue that was drawn by the RO. Half a centimetre of bolus was used to achieve the full dose to the skin.

C Planning axial CT showing the dosimetry with the dose wash option set to 44.83 Gy. The prescribed dose was 45Gy. This was accepted by the RO and treatment started.

D Halfway through treatment, the patient complained to the nurse of irritation within the external auditory canal (EAC). The nurse asked to see the planning axial CT showing dosimetry through the EAC with the dose wash option, this time set to 21.3 Gy, which is around half of the prescribed dose of 45 Gy. There is significant belling of the electron dose cloud into the EAC, which is lined by the epidermis, a hierarchically organised cell population. This is made worse by the air-filled EAC cavity as electrons can travel more freely in air than tissue. An acute radiation reaction of the skin in the EAC caused the symptoms.

E The RO was consulted and the patient was replanned using a centimetre of bolus. The revised planning axial CT shows dosimetry through the EAC with the dose wash option also set to 21.3 Gy. This effectively lifted the dose out of the EAC yet maintained, and possibly improved, the PTV coverage. From that point on during treatment, pink stuff bolus (black arrows) was put into the EAC. This bolus ‘soaked up’ any excess dose that did find its way into the air cavity of the EAC, further minimising dose to the skin of the EAC. The situation was explained to the patient and treatment recommenced.
Figure 3 Case study where astute nursing helped explain an unexpected acute reaction from MeV electrons.
MV photons: MV photons penetrate more deeply that SXRT and electrons. The exit beam in deeper tissues beyond the target must be considered. Figure 4A details a case study where astute nursing helped predict and explain the acute reaction from MV photons.

A A 70-year old man with multiple recurrent melanoma arising within a large resection scar on the left flank required radical RT to control any future recurrence. Screen shot A shows the prone planning CT scan with an axial cut through the abdomen. The blue line is the PTV. The dose colour wash is set to 47.5Gy (white arrow) which is 95% of the prescribed dose of 50Gy. There is good coverage of the PTV. This plan was accepted by the RO.

B An astute nurse asked the planning team to provide a plan with the lower bar of the dose colour wash set to half the prescribed dose. Screen shot B shows the same axial cut as A but with the lower dose range set to 25Gy, or half the prescribed dose (white arrow). Note that some of the large colon (red arrow) is included in the volume getting this dose. The large colon is lined by gut epithelium, a hierarchically organised cell population. A possible side effect of treatment could be diarrhoea. The nurse was able to predict this and educate the patient as to why it may occur. The nurse could then pay particular attention to whether this symptom was starting and could quickly institute appropriate care if and when it happened to ensure timely course completion.
Figure 4 Predicting acute reactions from MV photons using the planning dose colour wash.
Nurses can also explain and care for unexpected side effects by knowing the volume and dose from the computer plan. The case study in Figure 5A describes how this can be achieved.

A Antero - lateral photo of a fungating groin lymph node of Merkel cell carcinoma (MCC) of unknown primary in a female patient. MCC is particularly radio sensitive.

B Lateral photo of the case in A. The patient was to be treated with palliative MV RT in a spilt course approach to a total of 36 Gy in 12 fractions given in two phases of 18 Gy each. Each phase was to be delivered over two weeks, with a four week break between the phases to try and achieve some decrease in the GTV in the replan for the second phase. Splitting the course helped to decrease the acute effects and made transport easier for the family. This was thought safe to do as there is no evidence of accelerated repopulation in MCC.

C Screen shot C shows the supine planning CT scan with an axial cut through the groin. The pink line is the GTV. The blue line shows the PTV. Note the bolus (blue arrow) over the whole of the area. This ensures the full MV photon dose to skin. Note the use of ‘pink stuff’ bolus (red arrows) under the main bolus to stop air gaps so that the skin’s surface will definitely be irradiated.
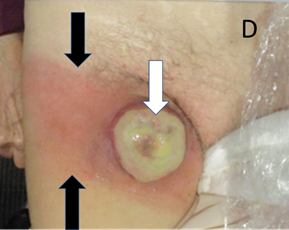
D An anterior clinical photo taken a week after the end of the first phase of palliative MV RT. There is a yellow exudate (white arrow) and the skin is red medially and laterally (black arrows) to the tumour mass. The nursing home where the patient resided was concerned that she had developed cellulitis.

E E shows a lateral photo taken at the same time as D, showing the same yellow exudate (white arrow) and redness of the skin (black arrows). However, the reaction was linear, reminiscent of the planar way of treating with the linac as described in article three.3 Also the patient was well with no systemic signs of infection, such as fever and tachycardia.
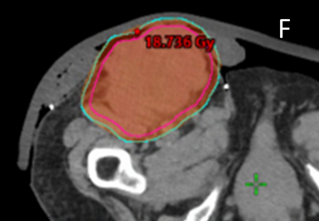
F Screen shot F shows the supine planning CT scan with an axial cut through the groin (as in C), but with the dosimetry with the dose wash set to the phase 1 prescription dose of 18Gy showing good conformity to the PTV. This plan was accepted by the RO.

G The astute nurse asked for the screen shot G which shows the same scan but with the dosimetry with the dose wash set to half the phase 1 prescription dose that is, 9 Gy. The 9 Gy covers the skin medial (white arrow) and lateral (red arrow) to the tumour mass where the acute reaction of erythema in normal skin was observed. The nurse was able to explain to the patient, the family, and the institution that the red skin was radiation dermatitis, and that the exudate was tumour necrosis. This was a radiation reaction and not infection, saving the patient from an unnecessary course of antibiotics. Nursing interventions could include a swab to rule out any underlying infection and education for nursing home staff about the tumour necrosis exudate and its appropriate management.
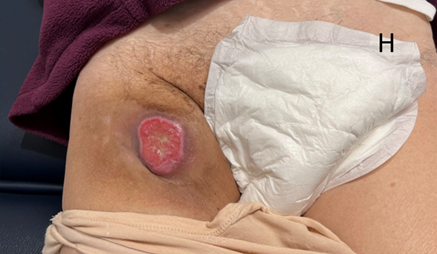
H Anterior photo H taken three weeks after the images in D and E. The tumour continues to resolve and there is now hyperpigmentation of the skin area previously thought to be cellulitis. This hyperpigmentation is significant compared to the contralateral unirradiated side and is typical of normal skin four weeks after a low dose of RT.

I G. Anterior photo I taken seven weeks after the completion of all RT showing that the Merkel lesion has resolved. The skin has closed (white arrow showing resolved ulcer). The hyperpigmentation continues to decrease (between the black arrows).
Figure 5 Case study – how a nurse could explain an unexpected side effect by knowing the volume and dose from the computer plan.
Caring for acute radiation reactions: As previously discussed, acute side effects are a sterile acute inflammation that take place in hierarchically organised adult normal cell populations that are within the high dose treatment volumes. The classic signs of acute inflammation are redness, heat, swelling, pain, and loss of function. Care during RT aims to keep the side effects to a minimum and tolerable so that treatment can be completed. The only effective way of stopping RT induced side effects is to stop or delay the RT, which may have an adverse effect on treatment efficacy and should be avoided at all costs. Patients should be educated on how to manage these acute changes with cool soaks and medication for pain as required. This ensures the continuation of treatment with some lessening of symptoms.
Systemic acute effects of RT: RT patients commonly complain of increasing tiredness and fatigue during a course of RT. The cause is unknown but is probably due to circulating inflammatory cytokines released as a result of the RT causing tumour cell death, and the body using energy to repair normal cells between the treatments. Therefore, the amount of fatigue will increase proportionally to the volume of the body being treated and the total dose delivered. Theoretically, systemic general treatments could include regular paracetamol. Paracetamol is an effective anti-inflammatory as well as being good for pain relief. Further research is needed. Adequate sleep is important as normal cells will only repair between fractions if they are rested. The patient can be encouraged to manage fatigue with rest and gentle exercise. Keeping a fatigue diary will help the patient to be aware of when they are at their mental and physical best during the day.
It is important to maintain weight during treatment as normal cells will only repair between fractions if they are well nourished. Fatigue can impact appetite and the ability to carry out activities of daily living, which especially impacts those who have to care for themselves. Any part of the gastrointestinal tract being irradiated can induce nausea. If symptomatic, simple anti-nausea medication like metoclopramide can help. Weekly weighing is essential. A decrease of two kilograms from the baseline weight can be remedied by adding 250 ml of a nutritional supplement beverage like Sustagen® three times a day. Loss of more weight may trigger the need for review by a dietician. Loss of significant weight, such as five percent of initial body weight or five kilograms, may trigger the use of a feeding tube, e.g., a nasogastric or percutaneous endoscopic gastrostomy (PEG) feeding tube. Some units do this prophylactically in patients at high risk of malnutrition. They are rarely needed for skin patients. The nurses need to follow the impact of interventions on weight loss in subsequent weeks to ensure compliance. Smaller, more regular meals that are easy to eat can help when patients are experiencing a reduced appetite or are becoming fatigued.
There can be many non-physical causes of fatigue, e.g., the daily grind of attending treatment for multiple weeks, a decrease in tension as the cancer is finally being treated, and the stress of dealing with many new people during the cancer journey. These are patient dependent but should be borne in mind if a physical cause cannot be found. Counselling via a support hotline or a referral for psychological support may then help.
Local acute effects of RT: RT is a localised treatment. Knowledge of what acutely reacting tissues are in the treatment volume together with knowledge of the entrance and exit beams is essential for predicting, explaining, and caring for skin RT patients. Many products are available to assist acute reactions and more regularly enter the marketplace. There is limited sound high level evidence and a general lack of consensus on the use of specific agents for the prevention and management of radiation induced skin reactions.10 The science behind the interventions will be explained here.
Acute local effects occur when acute inflammation arises in normal tissues in the high dose volume towards the end of a fractionated course of RT. Acute inflammation, especially erythema, is the normal healthy response to the cell killing effect of RT which helps normal tissue to repair. The increased blood flow associated with erythema brings the nutrients needed for normal cell repair. Although there is evidence that steroids are beneficial in decreasing RT acute effects when skin is not the target,11 stopping the acute inflammation per se with steroids may delay healing, and these should be used sparingly under medical supervision.
Most general interventions are aimed at symptom relief and include anti-inflammatory topical treatment like cool packs and soothing creams. Care must be taken to ensure that topical treatments do not worsen or add to the inflammatory effect of RT e.g., cold packs that are too cold or the use of tape on normal in-field skin. Cream application immediately prior to RT is discouraged as this may add a confounding bolus effect. The modern advice is to use a small amount of cream only prior to RT if warranted.12 Creams with a metal base, e.g., zinc and aluminium, may lead to unmeasured RT deposition in skin and should not be used but more research is needed.
Care of specific normal tissues that may be in a radiation volume
Skin: Skin is a hierarchically organised renewing adult cell population and so will exhibit acute and late effects. As detailed in article two,2 RT destroys stem cells in the hair follicles and sweat glands that create the natural moisture which keeps the surface dead cell layer together. A lack of this moisture leads to dry desquamation. This natural moisture needs to be replaced by applying a suitable moisturiser from the beginning of RT.
Moist desquamation occurs when there has been so much loss of skin stem cells that fluid from the dermis can be detected on the surface - the epidermal skin barrier has been broached. See Figure 13C of article two.2 Care needs to be taken to avoid an infection via this broken skin. When the skin is broken, best nursing practice aims to strike a moisture balance whereby healing is facilitated in a moist (not wet) environment.13 A moist healing environment enables the surviving normal cells to migrate across the wound surface to cover the breaks in the epidermis.14 Maceration may occur when excessive moisture is not adequately managed. This is destructive to the healing process and may compromise otherwise good tissue.15 Conversely, allowing the dressing to dry out and then taking the dressing off will remove new cells, delaying healing. Dressing technology has improved in recent years. Modern first-line interactive dressings are constructed of layers designed to minimise surface adherence and check exudate whilst maintaining a moist healing environment. They also provide outer protection against bacterial invasion.14 These dressings are therefore ideal tools for the radiation oncology nurse’s bag.
Hair bearing skin will suffer from temporary alopecia at 20Gy and permanent alopecia at 40Gy with standard fractionated RT. This may happen in the penumbra and patients may be so concerned about hair loss that a change in modality or technique becomes necessary.16 Penumbral issues in skin, like keratoacanthoma17 and hair pigment change18 have been reported.
Mucosa; Mucosa is another hierarchically organised renewing adult cell population that can be involved in scenarios such as irradiation of a cheek, nose, lip and the ano-genital regions. Patients can be reassured that these side effects will not last long as mucosa rapidly heals post RT, usually more quickly than skin.
When the oral cavity is involved, major and minor salivary glands can be affected. There are two sorts of salivary excretions: a watery clear fluid and a thicker creamy fluid. The cells that produce the former are more RT sensitive. Saliva therefore changes consistency over a course of RT when the salivary glands are in the high dose volume. Patients complain that their saliva is getting thicker, is more ‘stringy’, or that it has decreased in volume. Saliva is important for taste. As the penetration of food by the now thick saliva decreases, taste can change during RT. Patients can go off their food, impacting recovery between fractions. Regular mouthwashes and gargles with baking soda, two teaspoons in a half cup of warm water three times a day, cleans the mouth and aids ulcer healing.19 Teeth cleaning needs to be done gently with a soft toothbrush. Commercial mouthwashes can be soothing but the mouthwash must be free of alcohol.
Acute ulcers can be caused when RT involves the mucosa. Where the oral cavity is impacted by RT, nurses should be conducting weekly mouth assessments which include checking for oral candidiasis. Ulcer pain is related to fungal colonisation, hence the white colour. Sucking on amphotericin lozenges can help control this symptom. A viscous topical application of a local anaesthetic like lignocaine prior to meals can decrease ulcer pain during mastication. After application, patients need to be careful not to bite the ulcers, which can happen when insensate. Patients whose sensation is affected by local anaesthetics should be reminded to test the temperature of food prior to eating.
Sinus mucosa can be in the exit beam of RT to the forehead and face causing patients to complain of sinus congestion. This can present as pain, a runny nose and voice change, making the patient think they have a concomitant viral illness. Regular sinus saline washout can help during RT. Commercial products are available.
Denuded nasal mucosa can bleed. Bleeding from the nose can be helped by a commercially available preparation of vitamin E in a nut-based ointment. Bigger bleeds accessible via the nasal vestibule can be ‘sealed off’ with petroleum jelly on the little finger.
Perineal mucosa can be bathed often with warm salty water to keep it clean, aid recovery and resolve symptoms. There are commercially available devices that help, such as a Sitz bath.
Conjunctiva: Initially, the use of a simple lubricating eye drop or ointment can keep the area moist and soothe symptoms in the same way that moisturiser does for skin. Inflamed conjunctiva is painful and can be colonised by bacteria. If this starts to happen, antibacterial eye drops e.g., chloramphenicol, should be added. If the inflammation is significant, chloramphenicol ointment can be added at night. The patient can be reassured that this side effect will heal quickly after RT. If a certain area is painful and does not resolve quickly, a corneal ulcer may be present and an ophthalmic review, if not already undertaken prior to RT, is advised.
Ears: The EAC has a wax-producing epidermis that can be inadvertently included in the RT volume, especially during RT to the parotid gland. The wax may become more tenacious after RT. Patients may complain of decreased hearing, and examination reveals hard wax in the EAC. Continued topical medication post RT for thickened wax may be required. If still problematic, an otolaryngology review may be necessary.
Oesophagus: This organ is lined by mucosa and may be in the exit beam of a neck treatment for involved lymph nodes due to skin cancer. A softened diet, referral to a dietitian, and weekly weight checks are essential in this cohort. Topical therapy is difficult as the organ is designed to propel any contents distally. A commercially available suspension with aluminium hydroxide and magnesium hydroxide as antacids, and the local anaesthetic oxethazaine can help, particularly prior to meals. The approach aims to purely relieve symptoms. The best way to avoid a reaction is to exclude the oesophagus from the high dose volume through sensitive and expert planning.
Abdomen: The abdominal content is rarely involved in skin RT. However, RT to the upper abdomen, especially to the liver, can cause nausea and lack of appetite. RT to the lower abdomen can cause diahorrea, and this is cared for symptomatically. Over the counter anti-diarrheals like loperamide and bismuth subsalicylate can help control symptoms.
Bladder: The bladder is lined by transitional cell epithelium, another hierarchically organised renewing adult cell population. The inflammation caused by radiation is further irritated by the presence of urine, which is acidic, and which leads to dysuria. Drinking more water and alkalinising the urine with medications, such as potassium citrate, can help to ease the symptoms.
Limbs; Limbs can develop lymphoedema during RT. Measurement at baseline and throughout treatment can document any changes and trigger referral to a lymphoedema specialist during therapy before irreversible symptoms arise. When RT to the lower legs is being contemplated, referral to a lymphoedema specialist prior to RT, especially if extensive skin field cancerisation is being treated, is advised.
Caring for late radiation reactions: Nurses are rarely involved in caring for late radiation reactions but may be asked to do so. RT can cause leg ulcers,6 particularly in patients with comorbidities such as venous insufficiency, and conservative management may require months of dressings. Fistula, which is an abnormal connection between two parts of the body, when discharging onto an external surface may need regular dressing until closed.
An uncommon late radiation effect that skin RT nurses may see is radiation recall. This is an acute inflammatory reaction that occurs, often in skin, when certain anticancer drugs are given after radiation therapy. Usually there is a long break between the two therapies.20 The skin looks normal but seems to have remembered past radiation treatment. When another cytotoxic therapy is administered, the skin’s tolerance to the new treatment is exceeded in the area previously treated by radiation, and the skin reacts as if it is being irradiated again. This phenomenon will usually present in the medical oncology setting. It is therefore something that medical oncology nurses should be aware of and look out for in patients who have had previous RT.
Common questions asked of nurses during RT for skin
This section is included as nurses often have to respond to questions and provide education to patients.
What is cancer? Cancer is when the deoxyribonucleic acid (DNA) in the cell nucleus is damaged and suffers a mutation that releases cell division from the body’s control. The cell can then multiply, and the resultant mass or tumour can grow into a mass of abnormal cells called a primary tumour. This tumour can invade nearby local structures and destroy them, causing local symptoms. These mutated cells can even access the blood stream, lymphatic vessels, or nerves to cause secondary cancers or metastases elsewhere in the rest of the body, impacting overall survival.
What is radiotherapy? RT is a localised anti-cancer treatment that uses radiation to treat a volume of tissue comprised of abnormal cancer cells and normal cells. RT denatures the DNA of the cancer cells leading to cancer cell death. The normal cells in the same treatment volume can be preserved when RT is given correctly. A certain total dose of radiation to the volume needs to be given. This dose is determined by the disease being treated.
How does RT work? RT causes breakages in the DNA of cancer cells that leads to cancer cell death. Normal cells can repair the damage done to their DNA by fractionated RT. RT can therefore treat a mixed population of normal and cancer cells and destroy cancer cells whilst preserving normal cells. This leads to normal tissue conservation and hopefully improves survival. However, if too much RT is given at once, the repair capacity of the normal cells is swamped and they will also die. These dead normal cells are eventually replaced by fibrous tissue causing in skin hypopigmentation (increased pallor), scarring and telangiectasia (small blood vessels visible in the skin). To ensure the optimal survival of normal cells, RT is delivered in small daily doses called fractions.
Why so many RT treatments? The total RT dose is given in small doses so that normal tissue repair in maximised. This allows for optimal normal tissue conservation with better function and cosmesis giving better survivorship. How many treatments depends on tumour, treatment and patient factors. These are best discussed for each particular case with the prescribing RO.
Does RT hurt? RT does not cause pain during the application of each fraction. However, the dose of RT to normal tissues in the high dose region accumulates during the weeks of therapy, and this eventually causes acute inflammation, which can hurt. How much inflammation arises is influenced by the total administered dose and patient factors, including inherent individual radiation sensitivity.
What is the difference between RT and surgery? RT and surgery are both localised treatments. RT differs from surgery in several ways. Surgery removes both normal and cancer cells. RT destroys cancer cells and can preserve normal cells when given correctly. Surgery is also usually performed in one session. The tissue taken at surgery is usually sent to the histopathologist who generates a report which outlines the characteristics of the cancer and comments on whether it looks to have all been removed. As no tissue is removed with RT, no histopathology report is provided. Surgery and radiation therapy give roughly equivalent outcomes when used definitively for treating early skin cancer.21
Why would RT be used instead of surgery? RT is often used as an additional guarantee to surgery, e.g., when surgery has been performed but the histopathologist has discovered a positive margin, meaning that some cancer may have been left in the body. RT is also used for local control in situations where the patient is not a good candidate for surgery or declines surgery, e.g., the patient is unable to tolerate an anaesthetic or wants to conserve an organ or tissue. RT is a viable option as it can preserve normal cells.
What is the difference between RT and chemotherapy (CTh)? RT is a localised anti-cancer treatment whereas CTh is a systemic therapy; CTh goes all through the body. CTh can therefore cause symptoms away from the site of the cancer like nausea, alopecia, diarrhoea, and loss of white blood cells. Side effects of radiation therapy, on the other hand, are localised to the treatment area except for fatigue, a common RT side effect.
Will I lose my hair, have nausea, diarrhoea, or loss of white blood cells with RT? RT is a localised treatment and side effects will only be felt in the localised volume being treated. Hair loss will only occur to hair-bearing skin that is within the treatment volume, e.g., if the groin is being treated, some pubic hair may fall out. If the gut, bowel, or lots of bone marrow are in the treatment volume, then nausea, diarrhoea and loss of white blood cells may be experienced depending on the dose, but this would be unusual in skin RT.
What is planning? RT is a localised treatment that must be aimed at a localised volume of tissue in or on the body. The same volume has to be treated every day for the number of fractions prescribed. Planning therefore ‘maps out’ how and where the radiation dose needs to be applied. Importantly, it also considers the best way to position the patient to ensure that is reproducible for each treatment. This may require the use of immobilisation devices to decrease movement such as masks for head and neck treatments, vacuum bags for the rest of the body, and tape for keeping bolus and cut-outs in place.
What is bolus? Bolus is a tissue equivalent material that is mainly used to bring the full radiation dose to the skin’s surface, especially when using megavoltage treatment from a linear accelerator.
Summary of Article 4
This fourth and final article aimed to assist the nurse to apply the knowledge from the previous articles in order to predict, explain and care for the side effects that may arise during a course of skin radiotherapy. Acute effects, especially in hierarchically organised renewing adult cell populations, were explained. Late effects, such as fistula, radiation ulcers, osteoradionecrosis and radiation recall, were covered. Case studies were provided to guide the understanding of acute effects in relation to volume and dose with SXRT and MV elections and photons. A fundamental skill is learning how to understand the high dose RT volume from the RT prescription and plan. As a rule of thumb, to predict where and what acute side effects may arise during a radical course of megavoltage RT, the nurse can look for organs that have a hierarchically organised renewing adult cell population that are within the dose cloud of half of the RT dose prescription. These organs will suffer from acute side effects. The approach to care for these organs is outlined, and the common questions asked of nurses by skin RT patients are detailed, along with answers, in the article.
Understanding the mechanism of radiation induced skin reactions and their predictable nature in terms of onset and resolve can assist the nurse to make astute clinical decisions regarding their care and management. Some may think that this knowledge is beyond the remit of the nurse looking after skin RT patients; however, Figure 6 shows this is not the case.

Figure 6 Image of the surface of celebratory cake made by a nurse. The cake surface is 20 cm x 20 cm. This surface represents the dosimetry of a bilateral lower leg treatment for extensive skin field cancerisation complete with bolus, dose graticule (red arrow), and orientation cartoon (blue arrow).
This fourth article rounds off a four-part series aimed at assisting the radiotherapy nurse to better manage the side effects of RT skin patients. The first article focused on the role of the nurse within the radiation oncology department and the initial patient assessment. The second article revised the anatomy, physiology, pathologies and radiobiology of skin. The third described the skin RT prescription and plan, especially in relation to volume and dose. This fourth article aims to describe how the nurse, by applying the knowledge from the previous articles, can predict, explain, and care for the acute side effects that may arise. Hopefully, these scientifically based articles will assist and support nurses so that they can continue their great mission of caring for patients as a vital part of the skin RT team.
The authors would like to gratefully acknowledge the support of Xstrahl for making this series of four publications possible. We also wish to thank Aileen Eiszele of A&L Medical Communications for writing assistance and for overseeing the journal submission process, and Jack Fogarty for the graphic design of some figures. Finally, we wish to thank and compliment Jenny Kim on her cooking abilities.
All authors declare that there is no conflicts of interest.

©2022 Fogarty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.