International Journal of
eISSN: 2574-8084


Educational Review Volume 9 Issue 2
1Icon Cancer Centre, Revesby, New South Wales 2122, Australia
2Icon Cancer Centre, Concord Hospital, Concord, New South Wales 2137, Australia
3Strategic Investment and Clinical Care, Icon Cancer Centre, Queensland 4101, Australia
Correspondence: Gerald B. Fogarty, Icon Cancer Centre, Revesby, New South Wales 2122, Australia, Tel +61 (2) 87222800, Fax +61 (2) 87222801
Received: June 29, 2022 | Published: July 21, 2022
Citation: Fogarty GB, Mallon F, Athar A, et al. A scientific approach to skin radiotherapy nursing Article 2 - The anatomy, physiology, pathology and radiobiology of skin and its diseases. Int J Radiol Radiat Ther. 2022;9(2):56-67. DOI: 10.15406/ijrrt.2022.09.00325
Nurses play an important role in the care of patients treated with radiotherapy (RT) for skin conditions such as skin cancers. A series of four articles will aim to supplement general oncology nurse training by providing a scientific basis to better understand the skin and its diseases, the use of RT in skin, and the management of necessary acute skin RT reactions to achieve best patient outcomes. The first article focused on the role of the nurse within the radiation oncology department and the initial patient assessment.
This second article describes the anatomy, physiology, pathology and radiobiology of skin and its diseases. These concepts are fundamental to the understanding of how acute effects develop during treatment and how to best care for them.
The transition time of normal keratinocytes in the epidermis from creation to demise (10-14 days) is discussed as essential to understanding the timing of acute RT effects. The effect of RT on DNA, and the different effects of RT on normal cells versus tumour cells, which underscore fractionation and normal tissue conservation, are also discussed. Finally, this article addresses the late effects of RT in normal skin, accelerated repopulation, types of skin cancers and their relative radiosensitivities, and the effects of RT on benign skin diseases.
The third article will describe the skin RT prescription and plan, especially in relation to volume and dose; and the fourth will show how the nurse, by applying the knowledge from the previous articles, can predict, explain and care for the acute side effects that may arise during a course of skin radiotherapy.
Keywords: radiotherapy, radiobiology, superficial radiotherapy, volumetric modulated arc therapy, skin, skin cancer, nursing, review
This is the second in a series of four articles that attempts to provide a scientific approach to skin radiotherapy nursing. The first paper1 outlined the importance of the nurse as a member of the radiation oncology team and their role in patient assessment to ensure an optimal journey to treatment completion.
This second paper will revise the skin’s anatomy (structure) and its physiology (how it functions). It will then examine relevant skin pathologies (diseases) and describe how the skin can fail to function correctly. The article will then discuss what is now known about the radiobiology of skin; that is, how the skin and its diseases respond to ionising radiation therapy (RT). This knowledge is fundamental to understanding how acute, or short-term effects develop during treatment and how to best care for them.
The third describes the skin RT prescription and plan, especially in relation to volume and dose; and the fourth shows how the nurse, by applying the knowledge from the previous articles, can predict, explain and care for the acute side effects that may arise during a course of skin radiotherapy.
Table 1 provides a glossary of the common terminology used in this article.
Word |
Meaning |
Skin anatomy |
Structure of skin tissue. |
Skin physiology |
How the skin functions normally. |
Skin pathology or diseases |
How the skin is not functioning as it should. |
Benign disease |
Tissue growing under control of the body but functioning abnormally. |
Tumour |
An abnormal mass that is usually malignant. |
Cancer or malignant disease |
Tissue not growing under control of the body. |
Radiobiology |
How the tissue in question reacts to radiation. |
RT acute effects |
Side effects from radiation in normal tissue occuring during or within three months of treatment completion and usually dominated by acute inflammation. |
RT late effects |
Side effects from radiation in normal tissue occurring six months after treatment completion and usually dominated by chronic inflammation, especially fibrosis. |
Gray (Gy) |
Units used to measure the specifically absorbed dose of radiation in tissue. |
Standard fractionation |
A course of RT in two (2) Gy fractions at five fractions per week. |
Hypo fractionation |
When the fraction size is greater than 2Gy per fraction, usually with less fractions. |
Hyper fractionation |
When the fraction size is less than 2Gy per fractions, usually with more fractions. |
Fraction |
RT delivered during one treatment appointment. |
Table 1 Glossary of common RT terminology used in the treatment of skin diseases
Anatomy of normal skin
The skin is the largest organ in the body accounting for about 15% of an adult’s total body weight.2 It is composed of three layers: the subcutaneous fat, the dermis, and the epidermis (Figure 1). All layers vary in thickness depending on what body part is covered; for example, eyelid skin is thinner than skin on the back. The skin is continuous with the mucous membranes that line the body's internal surface; e.g., the vermillion border of the lip joins the mucosa on the inside of the lip to skin.

Figure 1 Artist’s impression of the anatomy or structure of normal skin. Skin is a self-renewing tissue. The inner most layer is the subcutaneous fat. The next layer is called the dermis. The dermis has a rich blood supply and contains the bases of the hair follicles and sweat glands. The outermost layer is an epithelial lining called the epidermis that sits on top of the dermis. The epidermis and dermis are separated by the basement membrane. Lying on the epidermal side of the basement membrane are the basal cells. These cells are very active and act as stem cells. They make new cells called keratinocytes. Basal cells can do this as they have a rich blood supply, being close to the dermis.
The inner most layer of skin is subcutaneous fat which lies on fascia or tissue which covers muscle and other deeper tissues. Blood supply to the rest of the skin goes through this layer. The next outer layer is the dermis. The dermis is made of connective tissues; for example, fibrous tissue, capillaries, lymphatic channels, and nerves. The dermis comprises the bulk of the skin and provides its pliability, elasticity and tensile strength through fibres made of proteins like collagen and elastin. Collagen is fibrous. One difference between the skin on the palm versus the back of the hand is that the dermis of the palm has more collagen. The dermis houses the capillary network that supplies the epidermis and skin nerves. Adnexal structures, such as the hair follicles and sweat glands, are structures that are continuous with the epidermis but anchored in the dermis, with the latter supplying blood and nerves. Small muscles in the dermis control the erection of hairs and therefore help to thermoregulate the whole body.
The epidermis is the outer most layer. This layer interfaces with the outside world. It is separated from the dermis by an important structure called the basement membrane. Lying on the epidermal side of the basement membrane are the basal cells. Basal cells receive a rich blood supply from the dermis and constantly make new cells called keratinocytes that are stacked on top in different layers.
Other cells around the basement membrane include the pigment producing melanocytes; merkel cells, which are remnants of a past sensory system and, amongst others, Langerhans cells that regulate immune skin reactions.
Physiology of normal skin
The three skin layers form an effective barrier between the body and its external environment. Skin allows the transmission of sensory information. It helps in the prevention of excess water loss. The skin also plays a role in thermoregulation by altering vasodilation, contracting the hair erection muscles, and with the subcutaneous fat layer varying in thickness at different parts of the body.
The epidermis is a self-renewing tissue. The basal cells of the epidermis act as stem cells. They have large active nuclei and keep busy making new cells called keratinocytes thanks to their position close to the dermis which provides a rich blood supply. Stem cells also line the adnexal structures such as sweat glands and hair follicles that are anchored in the dermis. Their progeny are specialised cells that make hair, sweat and the secretions that contribute towards skin moisture.
In the process of normal skin function, new keratinocytes made by basal cells migrate continuously towards the skin’s surface. The keratinocytes mature as they are pushed upwards and form a series of layers of differentiation. These layers are marked by progressively thinner cells. The nuclei become smaller and eventually disappear. The existing cells are constantly being pushed away from their blood supply by new cells arising from beneath. This move away from the blood supply causes the cells to die. As a result, the skin’s surface is lined by dead cells that are continually being shed and replaced by those from beneath. These dead cells are held together by the secretions of the adnexal structures.
The transit time of a keratinocyte from creation by a basal cell to shedding at the skin’s surface differs depending on the skin’s position on the body (range = 10 – 14 days). This process is increased and accelerated in disorders such as dandruff and following sunburn when the shedding of skin is so great that it becomes visible. This visible loss of skin cells, which looks like skin flaking, is called desquamation. It can also happen in some benign skin disorders, such as psoriasis. Psoriasis is a benign disease characterised by rapid cell turnover which is enough to result in the visible loss of dead surface skin cells.
Normal epidermis is somewhat like a cancer – it has a rapidly dividing stem cell population which makes lots of new cells that eventually die. The difference is that normal skin cells are under the body’s control, whereas cancerous cells are not regulated and grow at will. How much they grow is often determined by the available blood supply.
Pathology of normal skin leading to skin cancer
The high rate of cell proliferation in the basal level of the epidermis, with exposed and active DNA, coupled with exposure to external carcinogens, especially UV light, can cause a high rate of mutations in the DNA of these skin stem cells. Most of these mutations are repaired and some cause stem cell death. Rarely, these mutations can cause the stem cell to become independent of the body’s control, transforming it from benign into a malignant skin cancer.
In-situ disease: Mutant basal cells give rise to cells like themselves that can initially fill the whole epidermis. This is called in situ disease. In situ disease means that the basement membrane has not yet been invaded. Figure 2A consists of four stylised drawings that depict the function of normal skin and the progression to skin cancer and morpheaform change.
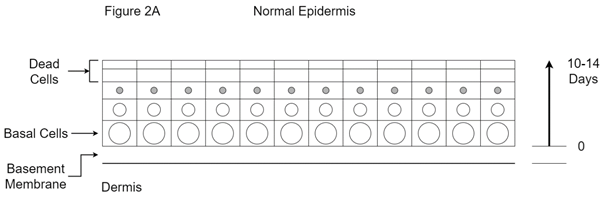
Figure 2A The normal epidermis is a self-renewing tissue. The stem cells or basal cells have large active nuclei and are busy making new cells called keratinocytes. They have a rich blood supply being close to the dermis. The new keratinocytes keep migrating towards the surface of the skin. They are pushed away from their blood supply by new cells coming from beneath, and so die. The skin’s surface is therefore lined with dead cells that are continually being shed and replaced by cells arising from beneath. Dead cells are held together by the secretions of the adnexal structures. The transit time of a keratinocyte from being created by a basal cell to being shed at the skin surface is between 10 and 14 days, depending on the skin site. This is normal skin function.
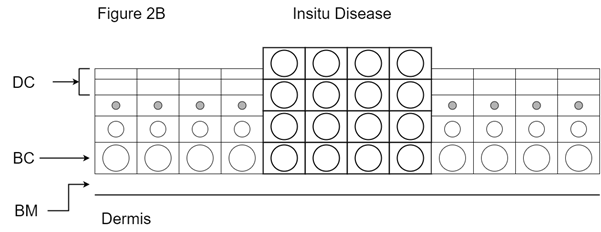
Figure 2B Explanation of in situ disease. The high rate of cell proliferation caused by the chronic irritation of UV light can lead to mutations in the DNA of basal cells in the epidermis, transforming the cell from benign to malignant. The large cells in bold are mutant basal cells. Mutant basal cells give rise to cells like themselves that can initially fill the whole of the epidermis. The filling of the epidermis can make the skin feel thick. Increased cell turnover may cause the sign of visible flakiness of skin. These cells can pull the blood supply with them from the dermis, causing visible erythema or redness in the affected areas. These areas can weep or even bleed with minor trauma and are often made worse if the patient is anticoagulated for other reasons. Importantly, the basement membrane is not yet breached in in-situ disease. DC is dead cells; BC is basal cells; BM is basement membrane.
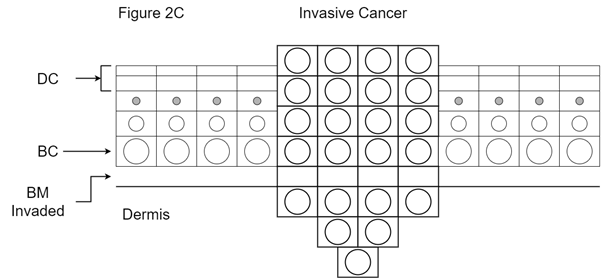
Figure 2C Explanation of invasive disease or cancer. Mutant basal cells are now able to invade through the basement membrane and access the dermis, with the ability to cause local destruction of the surrounding normal tissues. The malignant cells can also access dermal structures such as a) lymph channels, which can lead to regional lymph node metastases; b) blood vessels, leading to distant organ metastases; and nerves, which lead to perineural invasion and nerve problems. DC is dead cells; BC is basal cells; BM is basement membrane.

Figure 2D Explanation of morpheaform change. Mutant basal cells can migrate along the basement membrane and block off the pores of the skin through which exit the sweat ducts and hair follicles from the dermis. This gives a characteristic halo of lack of pores around the cancer. This halo needs to be treated otherwise field edge recurrence can occur. DC is dead cells; BC is basal cells; BM is basement membrane.
Figure 2 Stylised drawings of the function of normal skin and its progression to skin cancer and morpheaform change.
The increased cell growth of basal cells can cause itchiness and (dead) skin flaking, resulting in functional and cosmetic problems. These cells can pull the blood supply with them from the dermis which may present as visible erythema or redness in the affected areas. In situ disease can be symptomatic with itchiness, weeping and even bleeding on minor trauma, and this is often made worse if the patient is anticoagulated for other reasons.
Keratinocytic in-situ disease can be called Bowens disease, intra epithelial carcinoma (IEC) or cutaneous squamous cell carcinoma in situ (cSCC in situ). Sometimes this process can cover large areas. Areas over 50cm2 have been called extensive skin field cancerisation (ESFC)3 (Figure 3). Melanoma in situ can be called lentigo maligna or Hutchison melanotic freckle (HMF) (Figure 4).

Figure 3 A scalp showing typical features of keratocytic in situ disease with multifocal redness, thickness of skin and effacement of pores by morpheaform change. This area is larger than 50 cm2 and so can be called extensive skin field cancerisation (ESFC).
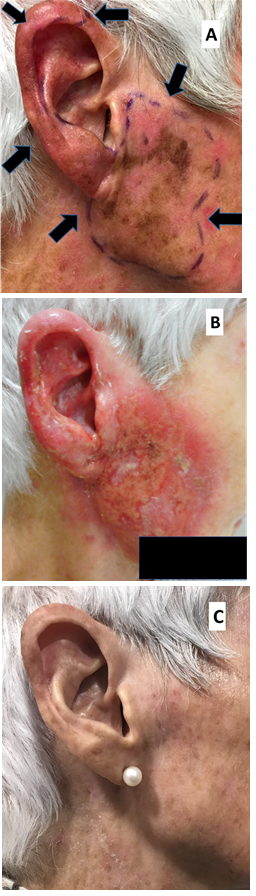
Figure 4 Melanotic in situ disease is also known as lentigo maligna (LM) or Hutchison melanotic freckle (HMF). This area of LM was mapped with reflectance confocal microscopy (RCM) and was found to be much larger that the visible extent. It was treated with definitive RT. Photo A is at RT planning. Arrows mark out the LM area. B is at the peak of the acute RT reaction. C is two years after RT with no sign, even on RCM, of LM.
Invasive disease or cancer: In situ disease can give rise to invasive disease. This is where the cells invade through the basement membrane into the dermis and can access different systems (Figure 2C). The rate of transition from in situ to invasive disease in the keratinocyte lineage has been quoted as being from 0.025% to 16%.4 In melanoma in situ, it has been estimated to be between 5% to 50% based on case studies which are the highest level of evidence available in this scenario.5
Skin cancers can be locally advanced because of morpheaform change. The mutant basal cells can migrate along the basement membrane and block off the pores of the skin through which the sweat ducts and hair follicle exit from the dermis. This gives a characteristic halo of a lack of pores around a cancerous ulcer. This halo needs to be treated to avoid field edge recurrence (Figures 2D and 5).
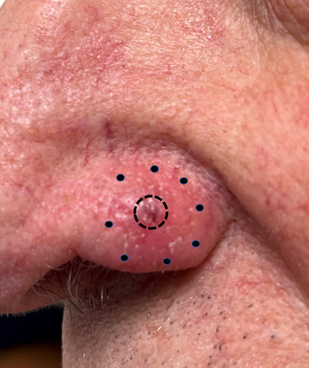
Figure 5 Example of morphaeform change. This is a photo of a biopsy-proven BCC on the nasal alar. The central ulcer is outlined with a full circle. Inspection with a good light shows that the skin pores are absent in the peri-ulcer skin outside the full circle. Obliteration of the skin pores outside this ulcer occurs due to morpheaform change and extends to the outer dotted line. Bimanual palpation of this area reveals thickened skin. It is a mistake to think that the cancer extends only to the edge of the ulcer.
Cancer stem cells are also called clonogens as they continually make copies of themselves. Cancerous cells can grow at a rate determined by the local blood supply. As the cancer grows, the new cells are eventually so far away from the blood supply that they die. Cancers can therefore have internal necrosis; that is, they can have a dead centre (Figure 6). Cells can even invade local structures like bone, making cure difficult.

Figure 6 Axial computed tomography (CT) scan of a large skin cancer on the chest probably arising from a lymph node metastasis. The tumour is outlined with white arrows. The centre, marked by a black star, is necrotic, having outgrown its blood supply.
Tumour cells can leave the bulk of the tumour, spread through the lymphatics found in the dermis (dermal lymphatics) to nearby lymph nodes, and cause lymph node metastases. A metastasis is defined as the development of secondary malignant growths at a distance from the primary cancer. Some sites in the body are more prone to developing lymph node disease, possibly because of the higher density of dermal lymphatics, e.g., lip and ear, making these sites high risk. This has been recognised in the most recent TNM staging5 (see below). Accessing the blood vessels can lead to metastatic disease at distant sites within the body, e.g., liver, lungs and brain.
Skin cancers can also access the dermal nerves and travel along the nerve causing nerve problems or palsies. This process is called perineural invasion (PNI). In sensory nerves, this can give rise to an altered sensation in the areas of skin supplied by the nerves called paraesthesia. A classic symptom is formication. This word comes from ‘formica’, the Latin word for ant. A sufferer describes the sensation of insects running around in the areas of skin supplied by the affected nerve. In motor nerves, PNI can give rise to poor nerve function which can occasionally produce symptoms and signs of decreased mobility (Figure 7A).
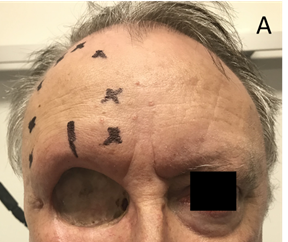
Figure 7A Perineural invasion (PNI). Sensory PNI. This man had an orbital exenteration for locally advanced adnexal carcinoma of the upper eyelid. He was referred for radiotherapy to an area of paraesthesia due to involvement of the supraorbital nerve by cancer. Black crosses mark the area of sensory loss. A line marks where the enlarged nerve was palpable as it entered the supra orbitual foramen.
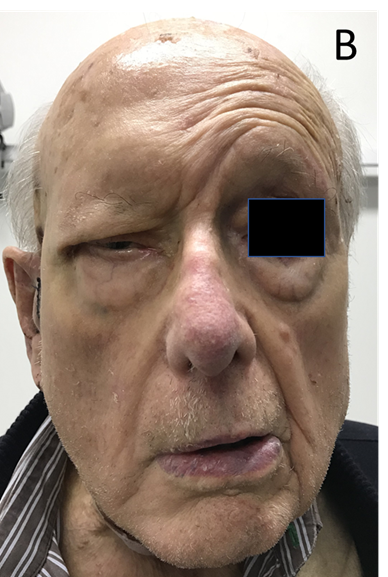
Figure 7B Motor nerve issues. Loss of movement to the right face following nerve injury of the right facial nerve with skin cancer. This was caused by surgery for parotid metastases due to skin cancer. PNI can present with similar facies.
Figure 7 Neurological loss due to skin cancer.
Staging of cancer: The staging of skin cancer is based on the accumulation of historical data derived from past experience and research. Staging is constantly reviewed as more data comes to light. Staging is important for a number of reasons. It determines the likely prognosis of a particular cancer and the current best treatment approach based on the treatment of similar tumours in the past. Staging is also important for research, especially comparative research where one treatment is compared to another within a randomised trial. Staging the cancers and then balancing the characteristics of trial participants and their tumour stages between the trial study arms ensures that each trial arm has similar participants. Any difference in efficacy between the different treatments under investigation can then be attributed to the drug or treatment being tested, not because of unfair or unbalanced patient selection.
Most staging is based on the tumour (T) – node (N) – metastases (M) classification or TNM.6 Staging is a numerical system used in cancer diagnosis to sum up how far a cancer has advanced. The increasing size and thickness of the tumour is captured by the T, the increasing number of lymph nodes involved is captured by the N, and the increasing number of sites involved by blood borne cancer is captured by the M.
Overall staging is determined by the different combinations that result from TNM staging. Stage I means the cancer is small and contains has no high-risk features. In stage II, the cancer still has not spread but is larger, or has other features that put it at high risk of spreading. In stage III, the cancer has gone beyond the primary tumour, e.g., it has spread to the lymph nodes. In stage IV, the cancer has spread to distant body sites or organs. Any tumour beyond stage I brings increased risk, and stage III and IV tumours can be immediately life-threatening. The staging of skin cancers, especially non melanoma skin cancer (NMSC), is not perfect due to a lack of data stemming from a lack of research.7,8 The current staging does not include the many reasons why some locally advanced cancers are referred for radiotherapy, e.g., PNI.
Importance of the immune system: Surveillance by the immune system usually eliminates cells that are turning malignant. Conditions that cause immunosuppression can lead to failure of the immune system and result in an increased incidence of skin cancer. Examples include some blood diseases, like chronic lymphocytic leukaemia (CLL), and medically induced immunosuppression to stop rejection of a transplanted organ. The immune system also becomes less effective with age, a natural process called immunosenesence. This means that cancer incidence, especially skin cancer, accelerates with age. These processes are more noticeable in people with white skin who have had a lot of sun exposure in their youth before the age of 20. White Australians are particularly at risk because of high UV radiation in Australia and an outdoor lifestyle.
Radiation effects on normal skin and the diseases of skin
Ionising radiation used in skin radiotherapy in Australia is so called as it causes chemical changes in the deoxyribonucleic acid (DNA) found in the cell nucleus. DNA is important for storing genetic information. Chromosomes are made up of DNA. They are responsible for storing the genetic information needed for life and are duplicated every time the cell enters the process of division. In mitosis or cell division, the chromosome duplicates itself into two chromatids. One chromatid (one copy of all the DNA from the parent cell) goes to each new daughter cell.
Radiation affects tumour cells and normal cells in different ways: RT causes double stranded breaks in DNA9 that can quickly repair. However, they can repair in a way that is not compatible with life or at least further cell divisions (Figures 8 and 9). Tumour DNA is more sensitive to RT than the DNA of normal cells. Normal cells can repair RT induced DNA damage better than tumour cells and they repair more precisely. Most repair in normal cells is completed within six hours of radiation exposure.
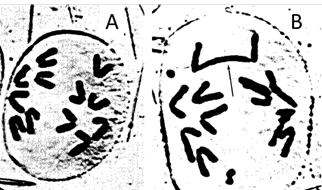
Figure 9 Nonsense recombination of chromones post RT. A. The cell on the left is undergoing normal cell division by mitosis. The chromatids have separated and are travelling to opposite ends of the cell, which will soon divide into two daughter cells. B. An irradiated cell. The chromosomes have suffered double stranded breaks and nonsense recombinations. This cell cannot divide. Photo courtesy of Dr Andrew See.
RT is fractionated to take advantage of the difference in repair between tumour cells and normal cells: Fractionation enables almost complete repair of normal cell DNA between each fraction, but this is not the case for the DNA of tumour cells. The normal cells continue to live whilst the tumour cells die. This is the main biological reason why RT is given in fractions. There are other reasons for fractionation – reoxygenation, redistribution, repopulation and radiosensitivity,10 but the main reason is the difference in repair between normal cell and tumour cell DNA following RT.
RT brings normal tissue conservation to cancer treatment: In a mixed population of tumour cells and normal cells, fractionated RT can selectively kill tumour cells but spare normal cells. Therefore, RT is very good at normal tissue conservation (Figure 10A).

Figure 10C Close up – The arrows indicate normal tissue that was inside the tumour. This normal tissue, and probably more, would have been excised with surgery to obtain an adequate surgical margin but has been conserved with RT.
Figure 10 RT is good for tissue conservation.
DNA repair in normal cells can be compromised in some situations: Normal cell repair is hindered if the amount (dose) of RT delivered during one treatment session, known as a fraction, is large. In such instances, the repair capacity of normal cells can become swamped causing some to die, which eventually can lead to replacement with scar tissue. This is one of the potential late effects of RT (Figure 11A).

Figure 11A Skin cancer site on the right upper lip treated with definitive RT which was given in large doses per fraction. The RT prescription may have been 36 Gy in six fractions at two fractions per week. Six Gy per fraction was therefore administered. Note the late effects of hypopigmentation, cicatrisation and telangiectasia.
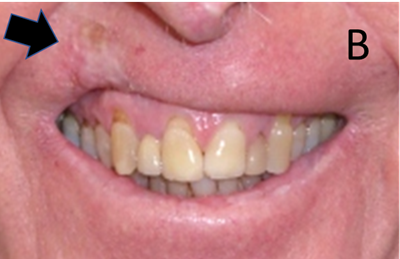
Figure 11B The same patient as in A. Fibrosis due to the hypofractionated course of RT has resulted in poor cosmesis which has negatively impacted this person’s smile.
Figure 11 Late effects in normal skin caused by a large fraction size.
Other issues that impede repair include a lack of nutrition. Normal cells need to be well nourished to repair, and so there is a heavy emphasis in RT on maintaining weight during treatment. In RT, routine nutrition screening is conducted for all at baseline, and patients at risk for malnutrition are referred to a dietitian. Weekly weight checks are important, and there is a low threshold to using nasogastric and percutaneous endoscopic gastrostomy (PEG) tubes. This ensures that the RT course can be completed without interruption.
Normal cell repair can also be impeded when a more rapid dose of RT is given or when treatment includes concurrent chemotherapy. For example, two fractions may need to be given in one day to make up the loss of a fraction due to a public holiday falling during the weekly schedule. With some cancers (not necessarily skin), chemotherapy is sometimes administered at the same time as RT to decrease the small amount of repair that can happen in tumour cells. An Australian based randomised controlled trial showed that concurrent chemotherapy was not needed in high-risk cSCC.11 Rarely, genetic syndromes can cause problems in normal tissue DNA repair,9 as can connective diseases.12
Radiation side effects in normal skin: Normal skin, especially when included in the target volume, will suffer side effects, and there is always normal skin in the treatment volume. Side effects are considered acute if they occur within three months of the completion of RT. Side effects starting six months after the end of RT are known as late effects. Consequential late effects13 are acute effects that have not been well cared for and have become a chronic problem. These side effects are described in the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.14
The grading of acute effects from RT in normal skin: RT causes acute inflammation in the skin. As more fractions are delivered, the in-field normal skin develops acute radiation dermatitis. The grades are depicted visually in Figure 12A.
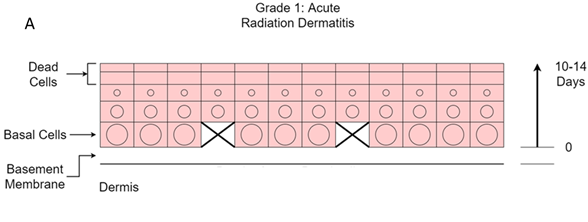
Figure 12A Grade 1. Erythema and swelling. Death of some basal cells leads to mild acute inflammation characterised by erythema and some swelling. The effects may be delayed due to the transit time of 10-14 days from the basement membrane to the surface. This starts to occur at 15-25 Gy in a standard fractionated course of RT to the skin.
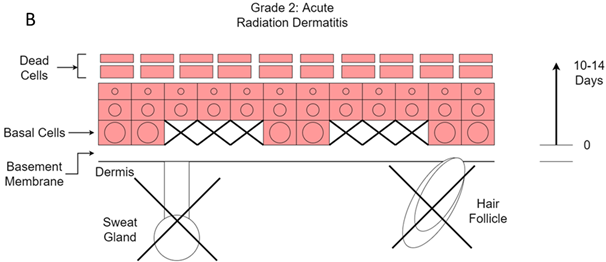
Figure 12B Grade 2. Dry desquamation. Death of more basal cells leads to more acute inflammation characterised by increased erythema and swelling. Destruction of sweat glands and hair follicles leads to loss of the natural moisturiser that is keeping the surface dead cells together. This leads to dry desquamation. It starts to occur at 25-35 Gy in a standard fractionated course of RT to the skin.

Figure 12C Grade 3. Moist desquamation. Some basal cells have been missing for some time and the cells they should have produced are not present, leading to holes in the epidermis through which fluid from the dermis can now pass to the surface. This starts to occur at 40 - 60 Gy in a standard fractionated course of RT to the skin.
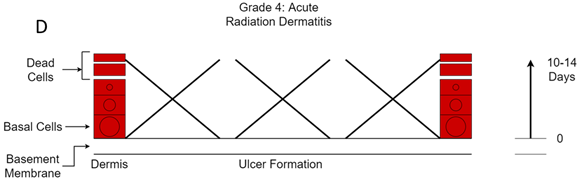
Figure 12D Grade 4. Acute radiation ulcer. So many basal cells have died that the loss of epidermis cannot be replaced. These ulcers can be painful. This starts to occur at over 60 Gy in a standard fractionated course of RT to the skin.
Figure 12 Chronological development of acute radiation dermatitis.
Grade one change is characterized by redness, or erythema, and swelling. This starts to occur at 15-25Gy in a standard fractionated course of RT to the skin given in two Gray (Gy) fractions at five fractions per week. This happens because radiation causes stem cell stress and death. Substances are released into the blood and the extracellular spaces that cause vasodilation. This inflammation is not caused by the cancer but by the radiation. At this stage there is redness in the normal skin.
Stem cells of the adnexal structures are also affected, and the production of naturally occurring skin moistures, like hair oil and sweat, cease. In hair bearing areas, hair begins to fall out at 15-20Gy.The skin can become hyperpigmented, especially in those with darker skin initially. This is because the pigment cells, called melanocytes, produce melanin, which is a DNA radio protector.15
The best way to delay the development of these effects is to artificially supplement the declining natural moistures by using regular moisturiser on the in-field normal skin from the beginning of RT. It is best to avoid metal-containing moisturisers as the metal may cause a greater deposition of RT in the skin. A steroid cream may help with symptoms but can also stop this protective mechanism and should only be used sparingly in this scenario.
Grade two change is when dry desquamation begins to take place. The skin looks flaky. This happens at 25-35 Gy of a standard fractionated course of RT to the skin. This happens because the layers of dead cells on top of the skin loosen. Normally, they are held together by the moisture produced by the adnexal stem cells. However, as the adnexal cells have died due to the radiation, which then results in moisture loss, the dead cell layers fragment and produce flaky skin. Continued moisturiser is the treatment of choice.
Grade three change is when confluent moist desquamation begins to take place. This happens when the dose of radiation during a standard fractionated course of RT to the skin reaches 40 – 60 Gy. It occurs due to the death of basal cells – as many have died, there are no cells overlying the dermis and fluid exudes. The skin at this point is open and liable to infection. Moist dressings to replace the covering on the dermis are now needed. Excess moisture at this stage creates maceration and further breakdown of tissue, so moisturisers should be ceased. The skin is fragile, and bleeding can be caused by minor trauma; therefore, one of the primary purposes of dressings is protection. Cellulitis will become evident if there is redness spreading outside the RT field, along with systemic signs like fever and tachycardia. If these signs appear, medical review is indicated. This effect may happen earlier in skin folds where the skin sparing effect of megavoltage RT may be lost. This is why treatment staff try to minimise the number of skin folds through which the incident megavoltage beam travels.
Grade four change is when so many basal cells have died that there is now necrosis of the skin causing ulcers. This happens at 60-70 Gy of a standard fractionated course of RT to the skin. These ulcers can be painful and are prone to spontaneous bleeding. It is prudent to have a haemostatic calcium alginate dressing on hand in case it is required during dressing changes. The ulcers may be slow to heal,16 or may never heal and require another intervention like surgical debridement. The aim of RT planning is to exclude normal skin as much as possible from the treatment volume so that it does not receive radiation.
Following RT, the skin usually repairs quickly in a few weeks with proper nursing care, but the adnexal structures take time to recover, if at all. The in-field skin may be left with no sweat glands nor hair. Hair bearing skin receiving 20 Gy by standard fractionation will be subject to acute hair loss; 40 Gy will result in permanent alopecia17 (Figure 13).

Figure 13 This man had post operative radiotherapy to the right parotid and neck with penumbra involving the right side of face. The photo was taken one month following RT. The skin has healed. Hair bearing skin areas receiving 20 Gy will have temporary alopecia - the hair will return over six months. Areas receiving over 40 Gy will have permanent alopecia.
Radiation effects in tumours: RT is damaging to tumours, which is obviously the desired effect. At quite low doses, even 20 Gy of a standard course, tumour necrosis with the production of what looks like pus, can be present. This necrosis has been caused by RT, not infection. RT kills but does not remove the tumour. The removal of dead tissue is achieved through the body’s normal homeostatic mechanisms. These may be defective and tumour resolution may take a long time, especially in people with comorbidities, which is why they were not deemed suitable for surgery in the first place. Following the definitive treatment of a particularly large tumour, a small mass may remain. This may be normal cell stroma that was supplying blood to the tumour and that survived RT. Biopsy of this lump while it is not growing is not recommended due to the possibility of fistula formation. Figure 14A is a case study that demonstrates the difference in radiation effects on normal skin and the diseases of skin.

Figure 14A Large fungating axillary node of cSCC. The lesion was inoperable due to patient factors and the patient was referred for definitive RT. The volume prescribed was 45 Gy in 15 fractions at five fractions per week. RT shorthand for this is 45/15/5. This is a hypofractionated course using 3 Gy fractions.
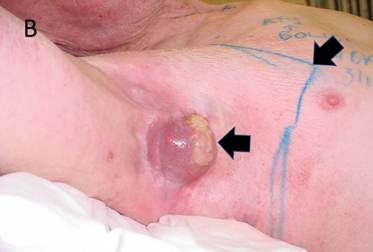
Figure 14B After 27 Gy. The tumour is exhibiting tumour necrosis as indicated by the horizontal black arrow. This looks like pus and is similar to pus as it is necrotic tissue. However, it has been caused by radiation, not infection. The diagonal arrow shows the RT field outlined in blue. In-field skin is getting full dose but shows no change at this dose.
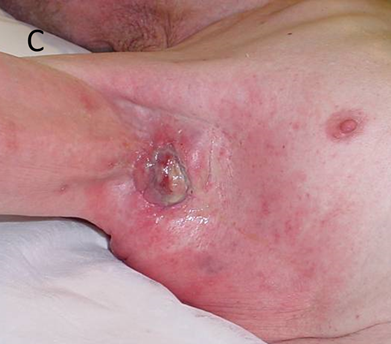
Figure 14C One week after 45 Gy in 15 fractions at 5 per week. The in-field skin is starting to develop erythema or acute grade 1 change. This is radiation dermatitis and not cellulitis. Antibiotics will not help.

Figure 14D Two weeks after 45 Gy. The skin is worse. It is now showing dry desquamation or grade 2 change, as indicated by the horizontal arrow, and moist desquamation or grade 3 change, as indicated by the vertical arrow. The reason why the reaction is worse two weeks after RT is that now all the change from RT is being exhibited. It takes 14 days for the keratinocytes that are not produced on day zero at the basement membrane level to be missed on the surface.
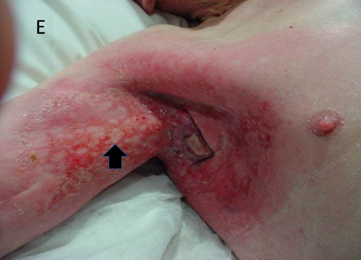
Figure 14E Three weeks after 45Gy. The skin is getting better. The vertical arrow is indicating islands of new skin that have grown from surviving skin stem cells. The skin is still open and needs expert nursing care to avoid infection. The redness is still confined to the treatment area so this is not cellulitis.
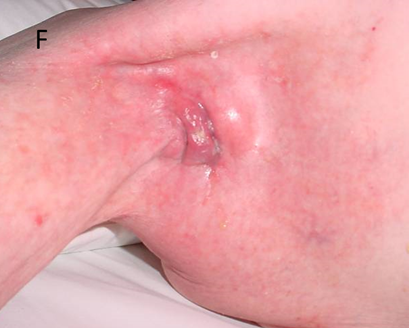
Figure 14F Six weeks after 45 Gy. The skin is healed. The tumour is still present but continuing to decrease in size. This is because RT kills the tumour but doesn’t remove it like surgery. It is up to the normal homeostatic mechanisms to remove the dead tissue.
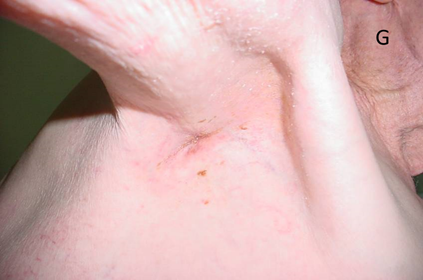
Figure 14G The tumour is finally gone at 18 weeks post RT.
Figure 14 A case study that demonstrates the difference in radiation effects on normal skin and tumour.
Late effects from RT in normal skin: Late effects are defined as occurring at least six months after the end of RT. They represent chronic inflammation. They usually arise because the fraction size is too great, causing the DNA repair mechanisms in normal tissue to become overwhelmed. This leads to loss of normal cells and replacement by fibrous or scar tissue. Clinical signs include hypopigmentation, telangectasia, cicatrisation, thinning and decreased skin elasticity (Figure 11).
Rarely, RT can cause a second malignancy. The rate is one in one thousand every ten years. The usual second malignancy in skin is a BCC.18 This is the reason why RT is preferably reserved for the elderly (over 60 years of age) as they have, on average, less time to develop a second malignancy. However, there is little data to support this practice which may be stopping younger patients from accessing effective tissue-conserving treatment.
Radiation sensitivity can vary between people, hence the reason for such a wide timeframe for when these effects can take place. There is anecdotal evidence that those with lighter coloured skin, blue eyes, red hair and a tendency to sun burn will have greater radiation sensitivity of their normal skin than others.19
Radiation sensitivity can vary between areas of normal skin. Chronically sun exposed skin is anecdotally more radiation sensitive than unexposed skin (Figure 15).
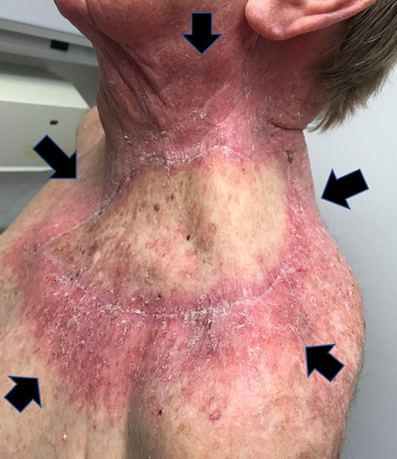
Figure 15 Radiation sensitivity can vary between areas of normal skin. Chronically sun exposed skin is anecdotally more radiation sensitive than unexposed skin. This man had a flap inserted into the left supraclavicular area followed by adjuvant RT. The photo is taken mid-course. All the skin is getting the same dose. Chronically sun exposed skin around the edge of the flap exhibits florid grade 2 acute change. The skin of the flap, taken from a less sun exposed area, barely exhibits grade 1 change.
Does the problem of accelerated repopulation occur in skin cancer? Accelerated repopulation20,21 is a problem, especially in mucosal squamous cell carcinoma of the head and neck (SCCHN). The theory is that as tumour cells are destroyed by RT, the surrounding surviving tumour cells lose the contact inhibition to growth that the now dead cells provided. The surviving tumour cells can then divide, and the tumour can grow during RT. One can think of this as the surviving tumour cells having room to grow into their surrounding space given that some tumour cells have now died. This tumour cell growth is, in fact, beneficial because the faster growing cells are now more sensitive to radiation.
However, because of increasing symptomatic side effects in nearby normal tissues, there may be a temptation to pause RT to enable symptoms to abate before resuming treatment. If this happens, the tumour can proliferate quickly during the break due to accelerated repopulation. Therefore, most RT courses are continuous, and symptomatic side effects are managed without a break.
Does this occur in skin cancer? There is retrospective evidence that skin cancer may not suffer from accelerated repopulation to the same degree, and that breaks can be taken without compromising cure.22,23 Prospective research is needed.
Types of skin cancers and their relative radiosensitivities: Skin cancers are usually found in sun exposed skin. A definitive diagnosis is achieved by taking a biopsy and sending it off to pathology. Previously, skin cancers were defined as belonging to two subgroups: melanoma or non-melanoma. The tendency now is to describe those that come from the most common skin cell, the keratinocyte, as being of keratinocytic origin. Cancers of keratinocytic origin are basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC).
BCC is the most common invasive keratinocytic cancer. It has a natural history of local invasion and is less likely to metastasise. A colloquial name for BCC is rodent ulcer. BCC does not respect anatomical planes and is best treated as soon as it is diagnosed (Figure 16). BCCs are relatively radiosensitive.

Figure 16 A BCC on the tip of the right nose that was neglected for 20 years. Arrows define the gross tumour volume (GTV).
cSCC is the next most common type of invasive keratinocytic cancers. cSCC has a greater tendency to metastasise than BCC. SCCs are relatively radiosensitive, and those of cutaneous origin even more so.24
Rarer skin cancers include those of the adnexal structures. These skin cancers are relatively radioresistant and need to be treated with a higher dose of radiation if treatment is to be successful. Melanoma, for example, arises from melanocytes that reside on the dermal side of the basement membrane. As a result, they lie closer to the dermal lymphatics and blood vessels. Melanoma is radioresistant, but some may be sensitive. Merkel cell carcinoma (MCC), another rare skin cancer, is quite radiosensitive. A test to determine an individual’s tumour radiation sensitivity is lacking, and more research is needed.
The indications for radiotherapy in the definitive, adjuvant and palliative settings are beyond the scope of this work but are covered in the relevant NHMRC guidelines.25 Suffice to say that the quality of the data used to establish the guidelines, especially the Australian contribution, could be better.7,8
Radiation effects on benign diseases of skin: RT has been shown to have efficacy in benign disease such as rosacea,26 psoriasis,27 hidradenitis suppurativa,28 disseminated superficial actinic porokeratosis,29 extramammary Paget’s disease,30 PTEN hamartomatous disease,31 and keloids.32 These diseases have something in common with malignant disease in that they share a rapidly dividing cell population (Figure 17A).
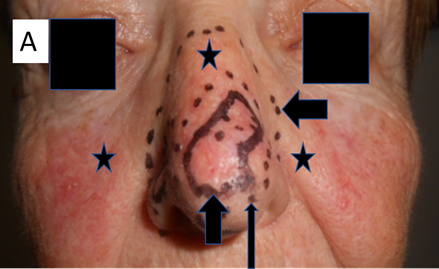
Figure 17A This lady had definitive RT for multifocal BCC of the nose as indicated by the vertical short black arrow. The horizontal short black arrow shows the volume being treated. The long black arrow shows the edge of the boost volume. Prior to RT, she had suffered from rosacea which had lasted for decades. The area of rosacea is indicated by the black stars. She was treated with a VMAT technique that treated all the convexity of the nose with a significant penumbra.
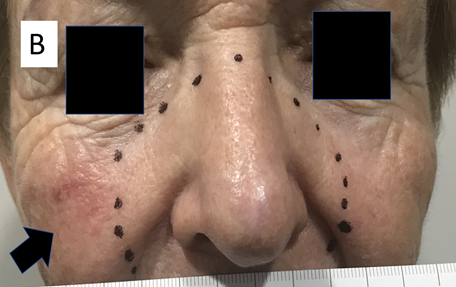
Figure 17B 12 months later, the same patient had a continuing complete response for her cancers. She also reported complete response of her in-field rosacea. The black arrow indicates an area of rosacea that was outside the penumbral field.
Figure 17 RT effects on benign disease.
There may be a temptation to decrease the RT dose as benign disease is considered less radioresistant than cancer. However, it would be wise to reflect that the reason why RT can selectively conserve normal tissue but kill tumour cells in a mixed population of tumour and normal tissue is due to the difference in cell repair. This is because tumour DNA is different to normal tissue DNA. In benign disease, DNA more closely resembles the DNA of normal tissue than the DNA of tumour tissue. It could therefore be argued that at least the same dose is needed as for malignant disease.33 More research is needed.
This second article has attempted to revise the anatomy and physiology of the skin. It then examined the pathologies of skin and focused on what is needed to understand the effect of RT on skin, especially from a nursing perspective. The transition time of normal keratinocytes in the epidermis from their creation to demise 10 -14 days later is crucial to understanding the timing of acute effects from RT.
The differing radiobiologies of skin and its diseases were then described. The effect of RT on DNA was explained as were the different effects of RT on normal cells versus tumour cells - the basis of fractionation and normal tissue conservation. Late effects from RT in normal skin were addressed along with accelerated repopulation, skin cancer types and their relative radiosensitivities, and the effects of radiation on benign skin diseases.
The third article will describe the skin RT prescription and plan, especially in relation to volume and dose; and the fourth will show how the nurse, by applying the knowledge from the previous articles, can predict, explain and care for the acute side effects that may arise during a course of skin radiotherapy, and so help patients through their radiotherapy journey.34
More prospective research is needed to provide answers to the many remaining questions about the radiobiology of skin and its diseases.
The authors would like to acknowledge the ongoing support of Xstrahl for making this publication possible. We also wish to thank Aileen Eiszele of A&L Medical Communications for medical writing assistance, and Jack Fogarty for medical illustrations.
None.

©2022 Fogarty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.