eISSN: 2576-4462


Research Article Volume 3 Issue 1
1Plant Secondary Metabolite Lab, Department of Biotechnology, Sant Gadge Baba Amravati University, India
2College of Horticulture, Dr. Panjabrao Deshmukh Agricultural University, India
Correspondence: Anita Surendra Patil, Plant Secondary Metabolite Lab, Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati 444602 (M.S) India, Tel 91-9881735354
Received: January 31, 2018 | Published: January 11, 2019
Citation: Patil AS, Kale AS, Patil SR. Studies on stages of somatic embryogenesis in Nothapodytes nimmoniana and its quantitative evaluation of camptothecin content. Horticult Int J. 2019;3(1):24 ? 29. DOI: 10.15406/hij.2019.03.00107
Nothapodytes nimmoniana J. Graham is an endangered medicinal plant species found in Western Ghats of Maharashtra, supposed to be the natural source of Camptothecin (CPT) an anticancer drug. Due to high utilization and export without giving many efforts on its conservation along with constraints of low seed germination, plant is said to be near to extinct. Thus Somatic embryogenesis is one of the methods to grow such plants in lab to field conditions. Somatic embryo formation will provide the information about the development and germination events and also aid in propagation of this species. Somatic embryogenesis was induced by leaf explants TDZ (Thidiazuron) plant hormone in various concentrations. But it was seen that TDZ (1mg/L) in Murashige and Skoog Basal salt media shows faster and better somatic embryogenesis as compare to other concentrations used with average induction of 60%.In histological examination of somatic embryos of N. nimmoniana various stages of somatic embryos were seen including globular, post globular, heart shape, torpedo shape etc. The HPLC analysis confirms the presence of CPT in invitro regenerated somatic embryos which were found to be 1.74058±0.08064 µg/g.
Keywords: Nothapodytes nimmoniana J graham, TDZ, somatic embryogenesis, camptothecin, histology, HPLC
mg/L, milligram/Liter; %, percentage; SE, somatic embryo; TDZ, thiodiazuran; MS, murashige and skoog; HPLC, high performance liquid chromatography; µg/g, microgram/gram; camptothecin, CPT
Nothapodytes nimmoniana Graham is an endangered plant species belongs to Icacinaceae family, which was reported to be widely grown in Western Ghats and North-east and is distributed in patches in India.1,2 The plant was confirmed to be chief source of Camptothecin (CPT),3 located in CPT, useful in cancer treatment.4 CPT has significant antitumor activity in mammalian cancer, AIDS chemotherapy, lung, breast and uterine cervical cancer.5–8 In last few years due to large scale cutting and illegal export plant material without giving much concern on its conservation has brought these plant under endangered category.9,10 Thus to meet the pharma-industrial demand and conservation view, micro propagation via leaf and stem of this plant has been reported,11,12 which seems to be positive approach for the conservation of this plant. As compare to Plant tissue culture, early seedling growth and seed germination process in N. nimmoniana is very slow because of seed dormancy.13 Gibberellic acid (GA3) treatment to seeds of N. nimmoniana increases in seed germination percentage.14
There are several reports about the potential of somatic embryogenesis in formation of new plantlets.12,15 Somatic embryogenesis has several advantages, including the efficiency formation of plantlets in few steps, reduction in labour, time and cost.13 The aim of this proposed work is to develop a protocol for induction of direct somatic embryogenesis for conservation and for deriving plant material for in vitro production systems. To best of our knowledge there are fewer reports to form somatic embryos in Nothapodytes nimmoniana.12 Thus, the study has been focused on the sequences of events microscopically and visually, which leads to somatic embryogenesis by using TDZ in tissue culture media directly from leaf explants and the validation of presence of CPT in them. In current research we are also looking for the occurrence of CPT quantitatively in the leaf generated somatic embryos.
Invitro somatic embryogenesis and histology
Plant materials
The seeds of N. nimmoniana were collected from place Chiplun from Western Ghats of Maharashtra India and are germinated according to our method.14 Fresh leaves were used for the plant tissue culture study. The leaves were washed under with tap water, and kept in 0.5% Bavistien for 2 minutes. These leaves were washed with laboline solution for 5 minutes followed by tap water and distilled water wash to remove soil, microbes and other external agent which interfere in tissue culture. The leaf explants were surface sterilized by 70% ethanol for 30 seconds which was followed by rinsing with sterile distilled water. In the next step explants leaves were treated kept in 0.2% HgCl2 solution for 1.0 to 1.5 min, which was again washed with sterile distilled water. The surface sterilized explants were cut into 1 cm×1.5 cm size and blotted onto filter paper folds.
Induction of somatic embryos from leaf explants
The surface sterilized leaf explants were used for inoculation on Murashige and Skoog (MS) medium. The MS basal medium was used for the somatic embryogenesis study, maintained at 5.8 pH by 1M NaOH or 1M HCl. The medium was supplemented with different six concentrations of TDZ (0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/L). The solidifying agent Clerigel (0.3%) was added in medium and the medium was autoclaved at 121oC at 15 lbs for 15 min. The tubes were allowed to solidify and checked for contaminations for 4 days. The experimental set I, II, III, IV, V and VI were inoculated with 0.5x1.0 cm sterilized leaf explants. The inoculated explants were maintained at 26°C temperature, white fluorescent light (1000 lux) for 12 h/day, with relative humidity of 40% in plant growth chamber (Remi).The inoculated tubes were kept under observation and after 25 days of inoculation the induction of somatic embryos from explants were recorded. The data at the end was statistically analyzed. To evaluate the embryogenic potential of the induced somatic embryoids was subcultures onto fresh medium corresponding to identical set. The medium was prepared as described above and cultures were maintained under the same conditions used for induction. The number of explants with embryogenic response and number of embryos per explants was recorded after 40 days of incubation. Occasionally, some explants tended to turn brown and had to be transferred to a fresh medium again. Few explants transformed and regenerated into white callus and maximally explants enlarged their size turn into full-grown somatic embryos.
Effects of TDZ on frequency of embryogenic response
For this experiment the leaf explant of N. nimmoniana plant was used and inoculated on the MS basal salt medium containing TDZ plant growth hormone ranging from 0.5-3.0 mg/L (0.5,1.0,1.5,2.0,2.5 and 3.0 mg/L). Twenty explants were inoculated in tubes for each concentration of TDZ. The explants with the positive embryonic response were inoculated in fresh media with their respective concentrations. The cultures were maintained under the same conditions used for determination of embryogenic callus frequency. The percentage of explants with well-developed somatic embryos formation was assayed after 40 days.
Histomicroscopical study
Histological studies of the somatic embryos generated in different concentrations, was done at different time intervals of the study. For the histological studies, the longitudinal sections (10–15 µm thick) of explants possessing somatic embryos were taken and fixed on clean slide using in FAA (Formalin 5% (v/v): Acetic acid 5% (v/v): Ethanol 90% (v/v), dehydrated in ethanol series and embedded in paraffin wax. About 10-15thin sections were cut and stained with (0.2%) safranine and observed under Carl Zeiss microscope. The histological analysis was performed to study developing stages of somatic embryos.
HPLC analysis of methanolic extract of Somatic embryo
Preparation of sample
Fresh somatic embryo of N. nimmoniana were crushed in the mortal pestle in HPLC grade methanol, taken in clean vials and percolated with 5 ml of methanol and vortexed for 2 minutes followed by sonication at 20 MHz at room temperature for 15min. The sonicated material was centrifuged and the supernatant was filtered by 0.25 micron filter and collected in another vial and further used for HPLC experimentation.
Preparation of calibration curve for Standard Camptothecin
Standard solutions of CPT in 20, 40, 60, 80 and 100 μg/mL were injected in HPLC column. The peaks were detected at 365 nm. Calibration curves of CPT were prepared by plotting peak area vs. concentration. The CPT concentration in various samples was obtained by plotting the values in standard calibration graph Figure 4.
HPLC analysis
The experimental sample solution somatic embryo for HPLC was filtered using 0.2 um syringe filter before injection. The instrument was purchased from Agilent Technologies (Model–1260 Infinity) and column used was ZORBAX Eclipse plus C-18 (4.6×100 mm, 3.5). Acetonitrile: Water (25:75) solvent system with flow rates 1ml/min was used. Detection of CPT was done at 365nm; elution gradient was isocratic elution with 10μl of sample. The flow rate was kept at 1.0 ml/min. DAD signal at 365 nm was kept for detection. Reference compound was commercially available CPT of Sigma Aldrich with concentration 1mg/ml.
Somatic embryo formation by leaf explant
A very simple protocol was optimized for the direct somatic embryogenesis from the leaf explant of N. nimmoniana. The leaf explant was cultured on the MS basal medium containing various combinations of TDZ which is one of the most efficient cytokinins like growth hormone. After 25 days of inoculation of explant on tissue culture media, induction was seen on each set, but better embryogenic growth was seen on set I, III, IV and VI. The embryos were formed on the leaf epidermal surface (Figure 1) (Figure 2) and were clear visible. They show several developing stages with less morphological differences. Some embryos were seen alone whereas some were found together in series with less morphological difference. The young somatic embryos were subculture on their respective multiplication medium, for the conversion of embryogenic mass to embryonic development.

Figure 1 Different stages of somatic embryos development (a-j) in N. nimmoniana [1a-c, Globular embryo stage, 1e-f, Leaf premordia; 1g-h, Callus formation from callus and 1i-j, Torpedo shape embryo and Shoot buds].
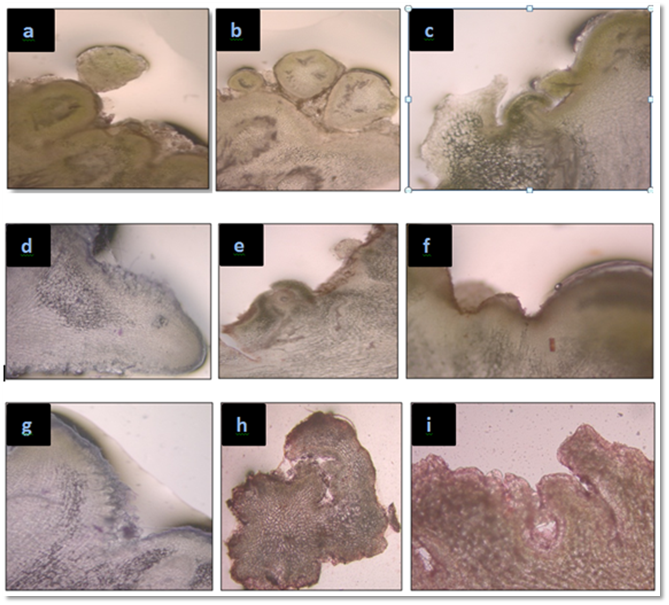
Figure 2 Microscopic study of different stages of somatic embryos development (a - i) in N, nimmoniana [GE , globular shape embryo, PGE, post globular shape embryo; S, suspensor, HE, heart shape embryo, SPM, shoot premordia, AM, apical meristem, CTD, cotyledonary shape embryo, TE, torpedo shape embryo and SB, shoot buds].
After 35-40 days these embryos were further developed, some embryos developed morphologically and some transformed into embryogenic callus (Table 1) (Table 2). It was found that the rate of embryogenic development is rapid in set I with 60.0 % response whereas Callus growth was faster inset IV with 70.0 % response as compare to other sets. After sub culturing the embryo converts whitish brown in color, which after further sub culturing converts into embryos with green color and giant shape. The histological studies of embryos were done after 60 days, up to which embryos were visibly green in color and circular in shape. In histological studies different stages of somatic embryos were seen under microscope, showing globular stage (early globular and post globular), shoot premordia, cotyledonary somatic embryos, apical meristem, torpido shape, Shoot buds and heart shaped.
Set |
Concentrations of TDZ(in mg/L) |
Explants inoculated |
Induction in SE |
% Response |
I |
0.5 |
20 |
12 |
60 |
II |
1 |
20 |
8 |
40 |
III |
1.5 |
20 |
8 |
40 |
IV |
2 |
20 |
16 |
80 |
V |
2.5 |
20 |
6 |
30 |
VI |
3 |
20 |
12 |
60 |
Table 1 Effect of variable concentration of TDZ on induction of Somatic embryos using leaf explants from N. nimmoniana
HPLC Analysis
The actual amount of CPT present in the samples was confirmed by HPLC using the standard CPT graph (Figure 3) (Figure 4). The CPT content in the 100 mg/mL methanolic extracts of somatic embryo was analysed and quantified by HPLC method. The chromatogram obtained shows homogenous peaks of CPT in standard and extract of somatic embryo with baseline separation at similar retention time 7.2 min. The exact retention time of test sample and standard CPT along with overlapping peaks confirms the presence of CPT in all samples (Figure 3). The estimation of CPT present in somatic embryo was performed on HPLC by preparing a calibration graph (Figure 4 Area v/s CPT amount in µg/mL) with Y-equation=1.33513x+1.97716 (error 0.00005). The amount of CPT present in test samples were calculated by putting the peak area value in automated generated calculator in the instrument. The results confirm that CPT extract of somatic embryo i.e. 1.74058±0.08064 µg/g with peak area of 15359 on chromatogram.
In vitro culture techniques have made possible to grow a rare and endangered plant in controlled conditions with the purpose of multiplication and conservations. In present study a protocol for direct somatic embryogenesis from the leaf explants of N. nimmoniana has been proposed, which seems to be quite beneficial for the multiplication of such endangered plant species. It has been reported that higher concentration of auxins is generally used for the somatic embryogenesis.16 Carrot cells shows the somatic embryogenesis in different stages in medium supplemented with 2, 4-D. But there is very less data available which explain the potential of somatic embryogenesis in multiplication of N. nimmoniana.12 Direct somatic embryogenesis has several significance in bio reactor for scaling up and synthetic seeds formation,17 along with plants formation without loss of parental characters.18 Somatic embryogenesis is also useful in forest development and conservation concept including woody plants, which has saved many plant species.19–21 TDZ is one of the most important tools for the somatic embryogenesis in woody plants like N. nimmoniana.22–24 Some earlier reports are available on direct and indirect somatic embryogenesis in N. foetida .15,25,26
TDZ has potential more than any other cytokinins in somatic embryo formation in N. foetida as it gives response in lower concentration as well.27 In this study out of 6 concentration of TDZ used in MS bassal medium, Set IV, with 2.0 mg/L was found best in terms of embrypgenic response. Also at same concentration the brown embryos after subculture turned in to green colored large embryo further showed maximum somatic embryogenic percentage. The observation are in similar with earlier report of dual nature of TDZ in differentiation of somatic embryos in pigeon pea plant.28 It has been also reported that TDZ promotes cell division and differentiation potential as well as induces embryogenic competence like cytokinins and auxins and shows better quality of shoot formation along with cytokinin like 6-Benzylaminopurine (BAP).23 Table 2 suggests that with variability in concentration of TDZ, shown to produce callus, induce somatic embryos formation and in particular concentration induce both somatic embrioids and callus. Such triple role of TDZ is reported in earlier report by Mundhara and Rahid,29 thus the exact activity of TDZ is still topic of debate and thus more prominent study is essential on the role of TDZ. Padmanabha et al.1 showed the general patterns of accumulation of CPT in N. foetida across individuals, plant parts, plant size and sex of plants, in the Western Ghats of India. CPT from N. foetida has been analyzed by HPLC, HPTLC, DESI-MS and 1H-NMR methods.30–32 In present investigation, CPT was analyzed by the HPLC method proposed by Patil et al.33 Among different Camptothecin producing plant species, N. foetida has the highest CPT accumulation.34 In our investigation, regenerated structure from leaf of N. foetida i.e. somatic embryo shows presence of CPT. The similar pattern variation in accumulation of CPT in different phenotypic variant has been shown by Patil et al.35
Set |
Concentrations of TDZ (in mg/L) |
SE inoculated |
Response of SE |
% Response |
I |
0.5 |
10 |
6 Somatic embryo |
60 |
II |
1 |
10 |
2 Somatic embryo |
20 |
III |
1.5 |
10 |
1 callus |
10 |
IV |
2 |
10 |
7 Callus |
70 |
V |
2.5 |
10 |
4 Callus and 2 Somatic embryo |
- |
VI |
3 |
10 |
3 Somatic embryo and 2 Callus |
- |
Table 2 Response of Somatic embryos after subculture in their respective media and hormone concentration
None.
The author declares there is no conflicts of interest.

©2019 Patil, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.