eISSN: 2576-4462


Mini Review Volume 2 Issue 2
1School of Biochemistry, Devi Ahilya Vishwavidyalaya Indore, India
2Shri Vaishnav Institute of Science, Shri Vaishnav Vidyapeeth Vishwavidyalaya Indore, India
Correspondence: Sunita Kataria, School of Bio Chemistry, Devi Ahilya University, Khandwa Road, Indore, 452001, Madhya Pradesh, India
Received: October 26, 2017 | Published: April 26, 2018
Citation: Kataria S, Guruprasad KN. Interaction of cytokinins with UV-B (280 -315nm) on the expansion growth of cucumber cotyledons. Horticult Int J. 2018;2(2):46-54. DOI: 10.15406/hij.2018.02.00025
The negative effect on plant responses are expected due to increased amounts of ultraviolet-B (UV-B) radiation reaching on the Earth’s surface by stratospheric ozone depletion. Cytokinins (CKs) are essential phytohormones in plant growth and development. Perusal of relevant literature reveals that CKs mitigate the oxidative damage caused by abiotic stresses like salt, drought, high temperature and heavy metal due to their antioxidant effects. However, very few reports are available on the interaction of CKs with UV-B stress on the expansion growth of the cucumber cotyledons. CKs induced the expansion growth of the excised cucumber cotyledons in the darkness. CKs induced expansion growth of cucumber cotyledons in darkness was inhibited by supplemental UV-B. UV-B radiation enhanced the level of oxyradicals like superoxide and hydroxide radicals in the excised cucumber cotyledons, which was evident by EPR spectroscopy. CKs like Zeatin, TDZ, BAP and FAP reduced the level of oxyradicals produced in the dark grown cucumber cotyledons, while promoting the expansion growth of the cotyledons. Production of oxyradical in UV-B exposed cotyledons showed that these oxyradicals might partially account for inhibition of expansion growth. Since considerable amount of oxyradicals were quenched by CKs at higher concentrations (10 and 20 µg/ml) it partially restores the inhibition of expansion growth caused by UV-B stress. It indicates that overproduction of oxyradicals by UV-B could not be entirely responsible for the inhibition of CKs induced expansion growth of cotyledons; which might be caused by some other physiological changes caused by UV-B irradiation in addition to the production of oxyradicals. UV-B is also known to inactivate protein inhibitor of peroxidase in cucumber cotyledons. This may reduce the antioxidants defence and enhance the damaging effects of oxyradicals for the growth of cotyledons.
Keywords: cotyledons, expansion growth, inhibition, ROS, UV-BPlants are sessile organisms and therefore must continuously adapt their growth and architecture to a constantly changing environment. Plants monitor changes in their environment and are able to memorize and anticipate these changes.1 Plants that perceive some of these changes as stress signals activate signalling pathways to modulate their development and to enable them to survive. The complex responses to environmental cues are to a large extent mediated by plant hormones that together coordinate the final plant response. In plants cytokinins (CKs) are a class of growth-promoting phytohormone regulating a wide range of growth and developmental processes.2‒4 CKs are a family of ligands synthesized enzymatically from adenine,5,6 which play a important role in various physiological events such as cell expansion and differentiation, seed germination, leaf and chloroplast senescence.7,8 Numerous evidences indicate that CKs have both positive and negative effects on stress tolerance. Prior to drought irrigation with CKs increased the tolerance of bean plants, but in maize and sugar beet, had no effect and a negative effect on tolerance respectively.9 Arabidopsis plants grown on media supplemented with cytokinins had a higher survival rate when exposed to freezing or dehydrating conditions than non-supplemented plants.10,11 At the earth surface the substantial reduction in the stratospheric ozone layer has led to a remarkable increase in the solar UV-B radiation.12 The depletion of the ozone layer has raised concerns over the ecological implication on agricultural production and natural plant ecosystems.13,14 Solar radiation in the UV-B range accounts for a minor percentage of the total solar energy, but still could be potentially harmful because in the cells these short wave lengths are capable of causing deleterious effects.15 Probably the genetic damages are most important effects of UV radiation on plants, because macromolecules such as DNA, RNA and protein have strong absorption at the level of 280-315.16 Moreover, negative effects of UV-B radiation can be significantly increased or decreased by a variety of interacting stress factors in the natural habitat.17 The effects of UV-B on plant’s vegetative growth are variable.18 but reductions in shoot length and leaf expansion were generally found.19,20 Plants are photoautotrophic organisms and thus light in particular is an environmental factor of utmost importance for plants.21 It was earlier observed that UV-B dosage alter reactive oxygen species (ROS) metabolism in cucumber cotyledon and leaves.22‒24
Studies have shown that exogenous application of hormones provides protection to plants against abiotic stress and increases crop yield.25‒27 Exogenous application of cytokinins has been reported to increase the stress-tolerance capacity of plants indicating a beneficial effect of CKs in the regulation of plants adaptation to environmental stresses. Thus several studies with abiotic stresses like heavy metals, drought, salt and high temperature have reported that CKs could be involved in regulating antioxidant defence, while information on regulation of antioxidant metabolism under UV-B stress by CKs is lacking. Taking into account the potential significance of CKs in alleviating stress, the present review contains an overview of the impact of cytokinins and UV-B on plants, together with their related defence mechanisms and the role of CKs to alleviate the UV-B stress. Cucumber (Cucumis sativus L.) is one of the most common vegetable species in the world and it has been frequently used as a model plant for cytokinins and UV-B studies because of its sensitivity to UV-B and CKs.3,28,29
Role of Cytokinins in plants
At normal growth conditions CKs are important phytohormones which play a key role in several aspects of plant growth, metabolism and development. For example for cotyledon growth and development, CKs are the main responsible phytohormones.30 CKs are compounds with a structure resembling adenine which promote cell division and have other similar functions to kinetin. Kinetin was the first cytokinin discovered and so named because of the compounds ability to promote cytokinesis (cell division). Zeatin the most common form of naturally occurring cytokinin in plants today was isolated from corn (Zea mays). In almost all higher plants as well as mosses, fungi, bacteria, and also in tRNA of many prokaryotes and eukaryotes CKs have been found. CKs concentrations are highest in meristematic regions and areas of continuous growth potential such as roots, young leaves, developing fruits and seeds.31,32 It is usually established that CKs are produced in the root tips and translocated through the xylem to shoots.33
By the alleviation of stresses such as salinity, drought, heavy metals and oxidative CKs are also able to enhance seed germination.34 CKs have been long known to regulate cell CKs are also able to enhance seed germination, growth and yield of plants.35,36 CKs have been long known to regulate cell division, differentiation as well as many aspects of plant development—including root growth, root/shoot branching architecture and vascular development.37,38 CKs are needed for the formation of leaf cells, bud formation and probably synthesised in the areas with meristemic activity in the plant stems.38 To regulate plant growth and development CKs actively interact with other phytohormones. It has been known that the interaction between cytokinin and auxin, another key growth promoting phytohormone, has an influential role in root development.39 In the cotyledons of Amaranthus mangostanus L. seedlings IAA had an antagonistic effect on the light-induced or cytokinin-stimulated accumulation of amaranthine. Cytokinins in darkness have been shown to induce, a number of processes which are normally controlled by light, including amaranthin synthesis, chloroplast development, and differentiation of leaves and cotyledons.39,40
To establish the relative biological activity of plant hormones compared with et al., bioassays are used. The role of cytokinin-induced cotyledon expansion has been studied by several groups. During germination, Ikuma and Thimann41first reported that CKs caused expansion of lettuce cotyledons. Similarly these effects have been found on excised cotyledons of other plants, including mustard,42 radish,43 watermelon44 and cucumber.45 To study the cytokinin-induced growth and related metabolism excised cotyledons have been widely used as experimental system.46 The cytokinin induced expansion growth in darkness was used as a bioassay for CKs by Banerji and Laloraya.47 It has been established that cytokinin-induced cotyledon expansion was dependent on nucleic acid synthesis.48 Thomas et al.49 found that when CKs aggravates absorption of solutes or production of reducing sugars, which results in growth promotion additionally from loosening of cell walls during zeatin-induced growth. Both cell expansion and cell division are involved in BAP induced cotyledon expansion in cucumber in which cell expansion contribute more to cotyledon expansion.50 The enlargement of excised cucurbita cotyledons is based both on cell growth and cell proliferation.51 During the life cycle of eukaryotic cells, microtubules are capable of performing various tasks which may relate to cell expansion and cell division.52,53 Li & Ma54 found that light enhanced the cucumber (Cucumis sativa L.) cotyledon expansion as compared with dark and benzylaminopurine (BAP) further enhanced the expansion of cotyledons. In cucumber cotyledon expansion, BAP treatment markedly increased the contents of endogenous cytokinins and induced α-tubulin gene expression.54
Effect of UV-B stress on plants
The solar radiation which strikes the Earth’s atmosphere also contains ultraviolet (UV) radiations besides visible light. Ultraviolet radiation (UVR) can be divided into three spectral regions (based on wavelengths) UV-C (200–280nm), UV-B (280–315nm) and UV-A (315–400nm). UV-C, the most dangerous, is completely absorbed by the stratospheric ozone layer in the atmosphere. UV-B radiation is not completely absorbed by stratospheric ozone layer; therefore, it reaches to the Earth’s surface and is harmful to living organisms.18 UV-B radiation is an important component of natural sunlight with a strong impact on terrestrial ecosystems.55,56 In general, the effects of UV-B radiation on plants can be broadly divided into two classes reflecting the function of the response firstly, UV-B damage causing heightened stress response that will help the plant to endure exposure to high levels of UV-B and secondly UV-B causing a photomorphogenic response in the plant, a non-damage response that establishes UV-B protection and modifies development.57 The phenotypic responses evoked in plants, ranging from hypocotyl growth inhibition, cotyledon expansion, phototropic growth and regulation of stomatal opening to the induction of UV protective secondary metabolites such as flavonoids and sinapic acid esters.58‒60 Elevated UV-B radiation produces a wide range of morphological and physiological damages to plant growth and metabolism, photosynthetic performance, DNA damage, repair and photo reactivation and chloroplast membrane components.61‒63 Protecting plants from UV-B stress the UV-B-absorbing compounds such as flavonoids are considered as a critical barricade.64
For changes in growth, general development and flowering, UV-induced changes in DNA and/or plant growth regulators are the probable molecular reasons. Several of these responses can be directly linked to effects of solar UV-B on key cellular components; nucleic acids, lipids, photosynthetic pigments and proteins.65 DNA lesions caused by UV-B photons may have inhibitory effects on plant growth.65 An elevated level of UV-B causes the production of reactive oxygen species (ROS) and activates the general stress signaling pathways.66 Furthermore, the UV-B-dependent formation of dimers between adjacent pyrimidines in DNA strands may be both mutagenic and genotoxic due to blocking the progress of DNA polymerase.67 As a consequence, the exposure of plants to high levels of UV can lead to cell death dependent on ROS signaling.68 Suchar & Robberecht69 found out that repair mechanisms could not solely prevent the UV-B radiation interference with the cell division; resulting in significant reductions in leaf growth and development. Staxén et al.70 found that the exposure of cells to UV radiation has been shown to lead to a delay in the onset of mitosis using Petunia hybrida protoplasts. The divisions that occur through leaf expansion were more vulnerable to inhibition by UV-B.71 Any changes in the concentrations of plant growth regulators influence the processes which are dependent on them.72
The specific UV-B photoreceptor called UV RESISTANCE LOCUS 8 (UVR8)78 through which plants can directly perceives UV-B photons.73 UVR8 induces UV-B acclimation and UV-B stress tolerance in the plants (Figure 1).74,75 UVR8 is a seven-bladed β-propeller protein that exists as a homodimer held together by interactions between charged amino acids.74,75 The UVR8 homodimer converts to monomers that initiate a signalling cascade after UV-B absorption, which ultimately leads to transcriptional regulation of target genes.74,76 A combination of protective as well as repair measures involve in acclimation to UV-B; including the accumulation of UV-B-absorbing substances in the vacuoles of epidermal cells, increased levels of antioxidants, protection of the photosynthetic apparatus and increased levels of DNA repair enzymes. The expression of numerous genes that strengthen photomorphogenic responses to UV-B which ultimately lead to UV protection and acclimation are specifically regulates by UVR8 (Figure 1).74,77 Besides mediating UV-B acclimation, UVR8 have a much broader effect on plant growth and development. UVR8 has also been implicated in UV-B-mediated entrainment of the circadian clock,78 hypocotyl growth inhibition,74 stomatal closure,79 phototropic bending,80 leaf development60 and osmotic stress81 and pathogen responses.82 Clarification of these mechanisms will ease the understanding of the interactive effects of solar UV-B radiation and other environmental factors on plant growth and ecological relationships.
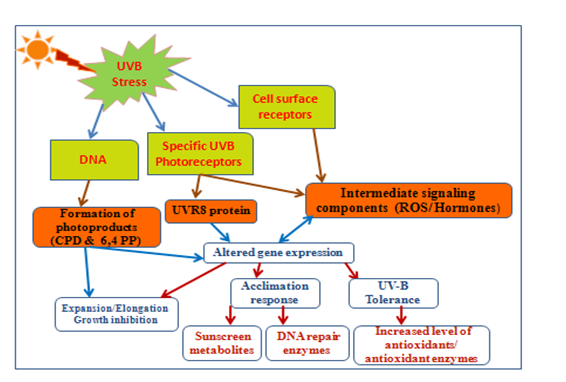
Figure 1 UV radiation activates UVR8 dependent photo-morphogenesis: increased level of UV-absorbing sunscreens gives acclimation response; increased antioxidative proteins can act as ROS scavengers; increased level of DNA repair enzymes can act on CPDs and 6-6 PPs lesions and caused overall growth inhibition.
Role of Cytokinins on abiotic stresses
CKs are hormones well known for its role in numerous aspects of growth and development, although abundant evidence also indicates that CKs functions in stress responses as well. Interestingly, previous studies have also reported that cytokinins can have protective effects against the damage caused by reactive oxygen species.83‒85 Several plant growth aspects and developmental processes, including cell division, apical dominance, chloroplast biogenesis, nutrient mobilization, leaf senescence, vascular differentiation, shoot differentiation, photomorphogenic development and anthocyanin production regulated by CKs.6,85 Increase in plant salt tolerance was reported after seed priming with Cytokinins.86 In plants CKs are often considered as ABA antagonists and auxin antagonists/synergists during various processes.87 In wheat plants by interacting with other plant hormones, especially auxins and ABA, cytokinins could increase the salt tolerance.88 CKs are generally considered to be antagonists of ABA, with the two hormones having opposing effects in several developmental processes including stomatal opening.89 cotyledon expansion and seed germination.90 A general view has come out that during stress; a reduction of CKs supply from the root alters the gene expression in the shoot and thereby brings out appropriate responses to ameliorate the effects of stress.91
Moreover, under stress conditions the observed reduction in endogenous CKs points towards the possibility that CKs levels could be a limiting factor under stress conditions. It can thus explain the fact that an exogenous application of kinetin resulted in increased growth of chickpea seedlings.92 Oxidative stress induced by salt stress is also detrimental to plants exposed to saline conditions, while due to its antioxidant effects CKs may mitigate salt-induced oxidative damages.93,94 Zhang & Ervin95 found that foliar spray of CKs could alleviate drought-induced leaf senescence of creeping bentgrass. During salt stress, exogenous CKs applications have been shown to enhance the salt tolerance in various plant species, such as eggplant (Solanum melongena), which displayed increased photosynthetic activity, biomass accumulation of roots and shoots and stem width along with decreased superoxide radical production rate and malondialdehyde (MDA) content.96 Tekchandani & Guruprasad2 found that Kinetin caused an enormous increase in the activity of peroxidase in UV-B exposed cucumber cotyledons. Ma et al.97 demonstrated that 6-benzylaminopurine effectively reduced salt-induced cellular damages by suppressing oxidative and ionic stresses in perennial ryegrass. The previous study indicated that application of BAP could increase endogenous contents of CKs such as zeatin ribosides (ZR), dihydrozentin riboside (DZR) and Isopentenyl adenine ribosides (iPA) and decrease ABA content in Kentucky bluegrass under drought stress.98 For Pisum sativum L. seedlings, 10μM of kinetin has been reported to enhance Mn tolerance and also increase seedling growth by improving ammonium assimilation and the antioxidant defence system.99
Interaction of Cytokinins with UV-B stress
Cell proliferation and expansion are two closely coordinated processes in dicotyledonous species for leaf growth.100,101 The integration of both external (environmental) and endogenous signals (phytohormones) are involved in the regulation of cell proliferation and expansion, eventually which determines the shape and size of the mature leaf and resultant in alterations in cell turgor and cell wall extensibility.102‒104 In the plants, UV-B radiation is a key environmental signal stimulating diverse metabolic or developmental responses.3,105 A range of morphogenic changes, including the inhibition of hypocotyl, stem and leaf expansion, stimulation of axillary branching in roots and shoots, and redirection of growth along the adaxial–abaxial axes induces by UV-B irradiance.105 According to Hóllosy65 a protective mechanism against damage caused by UV radiation was specifically interpreted as an increase in thickness.
In several plant species expansion growth of cotyledons is promoted in dark by CKs like Cucurbita pepo, radish and cucumber.43,47,106,107 Visible radiation also caused the expansion of cotyledons and leaves and this photoresponse is mediated by phytochrome.3,29,108 Photoresponses to UV-B (280–315nm) radiation has been in focus as UV-B radiation continues to increase at the Earth surface due to depletion of the stratospheric ozone layer. Reduction in whole plant biomass, plant height and expansion growth of leaves/cotyledons has been observed by elevated and ambient levels of UV-B radiation in several plant species.18,19,20,109 UV-B caused reduction in the expansion of cotyledons has been observed in soybean,110 Cucurbita pepo,111and Datura ferox,112 cowpea113 and cucumber.3,28,114,115 CKs like BAP, Furfuryl amino purine (FAP), Zeatin and thidiazuron (TDZ) promoted the expansion growth of cucumber cotyledons in the darkness and exposure of cotyledons to UV-B inhibited the CKs-induced expansion growth, which varied with the type of cytokinins used; inhibition was found more in FAP and zeatin compared to BAP and TDZ.115
Figure 3 shows the dramatic change in the cytokinins induced expansion growth of cucumber cotyledons by UV-B. According to Tekchandani & Guruprasad3 two mechanisms might be involved in the inhibition of cytokinins induced expansion growth of cucumber cotyledons by UV-B; it may have an inhibitory effect on cell division or reduce the cell elongation by the destruction of phytohormones. The enzyme cytokinin oxidase/dehydrogenase (CKX) inactivated the CKs;116 these enzymes catalysing the cleavage of their unsaturated bond. In previous studies it was shown that cytokinin levels and CKX activity are closely related.117,118 Irina et al.119 found that an inactivation of endogenous cytokinins may be involved in the reduction of leaf area, plant biomass and delayed development of pea cultivars after UV-B irradiation; they have suggested that this was not due to increased activity of CKX because the enzyme tends to be inhibited by UV-B irradiation. On the other hand, they have suggested that another way of cytokinin inactivation in the UV-B irradiated tissues might be involved in reduction of plant growth.119 By changing turgor pressure or cell wall extensibility UV-B irradiation may possibly reduce the cell expansion and Tevini & Iwanzik120 suggested that UV-B irradiation reduces the cell wall expansion by the direct oxidation of indole acetic acid. This might be the cause of the typical slight curling of the leaf surface, which is often seen under high UV-B irradiances.121 Recently, Vanhaelewyn et al.122 concludes that the effects of UV-B on hormonal regulation can be roughly divided in two, inhibition of growth-promoting hormones (like CKs, GA and auxins) and the enhancement of environmental stress-induced defense hormones. In addition, CKs crosstalk with HY5 in regulating flavonoid biosynthesis,123 suggesting that an interplay with UVR8 signalling may exist. Previous studies suggested that UV-B-reduced leaf expansion is completely due to UV-B-mediated inhibition of cell division in Lactuca sativa and Avena sp,124 A. Thaliana,125 cucumber cotyledons,126 parsley,127 wheat leaves,128 barley leaves,129 tomato hypocotyls58 and petunia leaf protoplasts.130 Repair of UV-B damage to DNA before replication and direct UV-B-induced oxidation of tubulin are mechanisms responsible for reduced cell division include oxidation of tubulin, which could delay microtubule formation.130
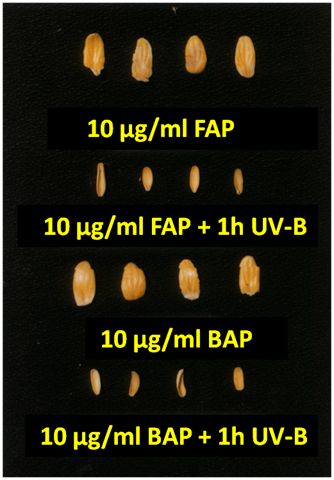
Figure 3 Inhibition of cytokinins induced expansion growth by UV-B (1h UV-B/ 24h Dark) in dark72h Dark).
Staxen & Bornman130 reported that the microfibrils, depolymerized when irradiated by UV-B and morphology of leaves altered by change in cell shape due to the disruption of the cortical microtubule network of epidermal cells. UV-B radiation may also affect the key stages of cell division through transcriptional repression of the genes encoding for a mitotic cyclin.130 The reduction in leaf size of UV-B exposed Arabidopsis plants has been associated to decrease in the cell expansion of the adaxial epidermal cells.131 Jansen105 found that the UV-B-induced reduction in plant growth, and more specifically in leaf area, has been attributed either to cell division or cell expansion or a combination of both. Some authors suggested that UV-B treatment reduced both the cell division and cell expansion in Pisum sativum,71 Triticum aestivum,132 & Trifolium repens.133 Fasano et al.81 found that UVR8-mediated accumulation of flavonoid and changes in auxin homeostasis are the basic mechanism for the reduced size of Arabidopsis rosette leaves and that UVR8 have an important role for integrating plant growth and stress signals.
Free radicals interact with the processes of hormonal regulation. Correlations have been found between superoxide formation and the increase in ethylene production.134 It has been shown that cytokinins inhibits the activity of xanthine oxidase, one of the cellular free radical sources, and also act as a direct free radical scavenger.135 Gidrol et al.83 suggested that the endogenous CKs might have a direct protective effect against oxidative stress, by scavenging superoxide radicals in addition to CKs role as a phytohormone. CKs are able to quench the superoxide anion as demonstrated in soybean seeds and E. coli.83 They have found that protective role of zeatin riboside may be due to a stoichiometric direct quenching of superoxide anions and may help to maintain seed viability by detoxifying reactive oxygen species. Yang et al.136 suggest that trans-Zeatin prevents UV-B induced collagen damage by inhibiting UV-B induced matrix metalloproteinases (MMPs) expression. It is well known that UV-B causes an oxidative stress by the production of superoxide radical (O2.-), hydroxyl radical (OH.) and hydrogen peroxide (H2O2). Jain et al.22 found a direct evidence for the production of oxyradicals by EPR spectroscopy in the cucumber cotyledons after UV-B exposure. Due to an oxidative stress caused by excessive production of active oxygen radicals UV-B suppress the expansion growth.115 Kataria et al.115 found correlation between quenching of oxyradicals by cytokinins (showed in Figure 4) with the promotion of expansion growth in dark grown cucumber cotyledons.
They also found the concentration response of FAP to UV-B exposure (5.6mW cm-2sec-1) in excised cucumber cotyledons, and showed that a considerable amount of the radicals were quenched at higher concentrations of FAP (64.4% at 10µg/ml and 65.5% at 20mg/ml) (Figure 4). It indicates that the oxyradicals might partially account for inhibition of growth in the cucumber cotyledons although the quenching did not result in any reversal in the inhibition of expansion growth.115 They have suggested that inhibition of cytokinin induced expansion growth by UV-B might be caused by other physiological changes induced by UV-B in addition to production of Oxyradicals. Tekchandani & Guruprasad2 found that the inhibition of cytokinins induced expansion growth of cucumber cotyledons by UV-B is accompanied by the increased activity of peroxidase and they have suggested the existence of an inhibitor of peroxidase which is susceptible to UV-B. Inactivation of peroxidase inhibitor may have an important role in higher plants to provide the protection against UV-B stress.28 Of the various defence mechanisms available to the plants, cucumber cotyledons have preferentially adapted the enormous increase in peroxidase as a stress alleviating mechanism when exposed to UV-B.2,28,137,138
Figure 4 Quenching of oxyradical by different cytokinins- Zeatin, TDZ, BAP and FAP in A) Chemical system generated by 1µl Potassium super oxide (KO2) + 100µl Phosphate saline buffer (PBS) + 5µl Phenyl N-t butylnitrone (PBN, a spin trapping agent) + required concentration of cytokinins, B) in tissue system cotyledons expansion growth in dark and after UV-B exposure. (Std.- control without cytokinins). For tissue system 100 mg of cotyledons were homogenized in 1.0 ml phosphate saline buffer (pH 6.0) containing 100 mM ethylenediaminetetraacetic acid (EDTA), 100 mM diethyldithiocarbamaic acid (DDC, a superoxide dismutase inhibitor) and 500mM phenyl N-tert-butylnitrone (PBN, a spin trapping agent+ required concentration of cytokinins).
In conclusion, the reduction in expansion growth of cucumber cotyledons was found to be due to an effect of UV-B on the rate and duration of both cell division and elongation. A lot of work has been done to determine the cytokinin levels under the water stress, high salinity and flooding. Thus when plants are exposed to various types of stress their hormone levels undergo significant and rapid changes. These changes result in the regulation of various physiological processes such as membrane permeability and water potential, protein synthesis and degradation, photosynthesis and respiration, and enzyme regulation. Various structural and morphological changes are also elicited indirectly by these sudden and dramatic changes. The role of inhibitor of peroxidase in this regulation of expansion growth assumes importance as a stress regulating factor. The inhibition in growth of the cotyledons may be the result of this stress management since the prevention of oxidative stress by the photo oxidants produced by UV-B may dominate the response of the cotyledons (Figure 2).
None.
The author declares that there is no conflict of interest.

©2018 Kataria, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.