eISSN: 2379-6367


Research Article Volume 12 Issue 5
1Faculty of Pharmacy, Al-Quds University, P. O. Box 20002, Abu- Dies, Palestine
2Al-Quds Bard College, Al-Quds University, Abu-Dies, Palestine
3Life Sciences Department, College of Science and Technology, Al-Quds University, Abu-Dies, Palestine
Correspondence: Mutaz Akkawi, Life Sciences Department, College of Science and Technology, Al-Quds University, Abu-Dies, Palestine, Tel ++972-2-526435785
Received: August 29, 2024 | Published: September 17, 2024
Citation: Abu-Lafi S, Zagharneh M, Abu-Remeleh Q, et al. LC-MS analysis of Urtica urens water extracts: active ingredients and their impact on beta-hematin formation. Pharm Pharmacol Int J. 2024;12(5):173-177. DOI: 10.15406/ppij.2024.12.00448
This study investigates the potential antimalarial efficacy of aqueous extracts from various parts of dwarf nettle (Urtica urens)-leaves, roots, and stems-by examining their ability to inhibit beta-hematin formation. The findings indicate that extracts from the roots and stems exhibit minimal antimalarial activity, while the leaf extracts show considerable promise. When the leaf extract was diluted, it maintained its antimalarial activity at concentrations up to 50%, but effectiveness decreased with further dilution. This decline may be attributed to the reduced concentrations of the active compounds present in the water extracts. The leaf extract was effective at concentrations ranging from 1 mg/ml to 0.5 mg/ml, but lost its activity at 0.3 mg/ml, likely due to inadequate levels of these compounds at this level. LC-MS analysis identified key flavonoids in the leaf extract, including flavanols such as Myricetin 3-O-rutinoside, Isorhamnetin 3-O-glucoside 7-O-rhamnoside, Kaempferol 3,7-diglucoside, Kaempferol-3-O-rutinoside, Rutin, Apigenin 7-O-diglucuronide, Kaempferol 3-O-(6''-acetyl-galactoside) 7-O-rhamnoside and flavanones such as Luteolin 7-O-diglucuronide. Examining their chemical structures offers insights into how these flavonoids might interact with heme, thereby enhancing our understanding of their antimalarial potential and supporting their consideration as candidates for malaria therapy.
Keywords: Dwarf nettle, annual nettle, Urtica urens, antimalaria, beta-hematin, chloroquine, hemozoin, UHPLC-MS
Malaria continues to be a major global health issue, despite significant progress over the past twenty years. The 2023 World Malaria Report by the WHO highlights a critical moment in the fight against this deadly disease.1 Since 2000, substantial investments have significantly reduced malaria deaths and cases. However, recent years have seen slower progress due to new challenges such as disruptions from COVID-19, increasing resistance to drugs and insecticides, and ongoing crises in malaria-endemic areas. In 2022, there were around 608,000 malaria-related deaths and 249 million cases worldwide, showing a slight decrease in deaths from 2021 but an increase compared to pre-pandemic levels.1 Sub-Saharan Africa remains the epicenter of the malaria burden, with Nigeria accounting for nearly a third of global cases and deaths. The disease predominantly affects young children, pregnant women, and displaced populations. In 2022, malaria-endemic countries distributed 345 million rapid diagnostic tests (RDTs) and 242 million Artemisinin-based Combination Therapy (ACT) courses, the standard malaria treatment. Despite these efforts, treatment-seeking rates remain relatively low. Surveys from Sub-Saharan Africa indicate that only 66% of individuals sought treatment for their most recent fever between 2015 and 2022, a slight increase from 65% between 2005 and 2011. However, among those who sought treatment, the use of diagnostic tests and ACT has notably risen.1
Malaria, caused by Plasmodium parasites transmitted by Anopheles mosquitoes, has seen a concerning increase in drug resistance, particularly against widely used ACTs. The rise of drug-resistant strains of Plasmodium falciparum, the most dangerous malaria parasite, emphasizes the urgent need for new treatment strategies.2–4 While ACTs have revolutionized malaria management, their declining effectiveness against resistant strains highlights the necessity for new antimalarial drugs. The spread of these resistant strains poses a significant risk of increased morbidity and mortality if not addressed promptly.
In response, researchers and pharmaceutical companies are focusing on discovering and developing new antimalarial compounds. Advances in drug discovery, coupled with a deeper understanding of the parasite’s biology and resistance mechanisms, are crucial for finding and implementing new treatments. Ensuring access to effective medications is essential for maintaining progress in malaria control and achieving eventual eradication. The fight against malaria is ongoing, and continuous research and development are critical for overcoming drug resistance and ensuring effective treatments for future generations.
The search for new malaria treatments increasingly turns to natural sources, recognizing their potential in addressing this persistent global health issue.5–8 Historically, effective antimalarial treatments have often come from natural products, such as quinine from the cinchona tree and artemisinin from the sweet wormwood plant.9,10 These natural compounds have significantly influenced malaria treatment strategies and inspire further exploration into nature’s potential.
Beta-hematin, also known as hemozoin, is a polymeric byproduct formed from the breakdown of hemoglobin by malaria parasites within the food vacuole of Plasmodium species. This compound is vital for the parasite’s survival as it detoxifies the free heme released during hemoglobin digestion. Antimalarial agents that target beta-hematin work by disrupting its formation or stability, which impairs the parasite's ability to manage oxidative stress and survive. In summary, these agents inhibit beta-hematin formation by preventing the polymerization of heme into hemozoin, causing toxic heme to accumulate in the parasite's food vacuole, disrupting cellular processes, and leading to the parasite’s death.5–8
Amongst Urtica species, Urtica urens, commonly known as dwarf nettle or annual nettle, belongs to the Urticaceae family and the genus Urtica. This plant is noted for its diverse range of natural products that contribute to its medicinal properties.11,12 It contains phenolic compounds, including flavonoids (e.g., 3-O-glucoside, quercetol 3-O-glucoside, kaempferol 3-O-glucoside, isorhamnetol 3-O-rutinoside, and quercetol 3-O-rutinoside) and phenolic acids (e.g., caffeic, ferulic, caffeylmalic, chlorogenic, and sinapic acids), as well as alkaloids like histamine and serotonin, and tannins, which give it astringent qualities.11,12 Additionally, it has essential oils with aldehydes and terpenes. These natural products support its traditional uses, including anti-inflammatory, antigout, immunomodulatory, antirheumatoid arthritis, antioxidant, antiviral, antimicrobial, anticancer, nephroprotective, diuretic, and antihistamine effects, making it therapeutically valuable.11,12
This study uses a semi-quantitative method to investigate how aqueous extracts from wild Urtica urens inhibit beta-hematin formation.13 Additionally, the UHPLC-PDA-ESi-MS was used to analyze and identify the bioactive phytochemicals present in a standard aqueous extract of dwarf nettle.
Plant material
Leaves, stems, and roots of dwarf nettle were collected from the Alzahiriya village in Hebron, Palestine. After separating these plant parts, they were washed with distilled water. The samples were then air-dried at room temperature, away from direct sunlight, for about two weeks. Once dried, they were ground into a fine powder and stored in a refrigerator.
Chemicals
Glacial acetic acid was sourced from Fluka, while ethanol (EtOH) was purchased from Merck (Germany). Dimethyl sulfoxide (DMSO) with 99.5% purity, chloroquine diphosphate salt, sodium acetate with 99% purity, and hemin chloride were all acquired from Sigma-Aldrich.
Extract preparation
To prepare the extracts, 2 grams of dried powder from the dwarf nettle leaves, stems, and roots were separately soaked in 150 mL of distilled water for 20 minutes. The mixtures were left at room temperature, then filtered and concentrated using an IKA WEREKRV06-ML rotary evaporator. After achieving the desired concentration, the extracts were freeze-dried with a Labconco freeze dryer until they reached a constant weight. The resulting dried extracts were stored in a refrigerator for future use.
Screening for antimalarial activity
The antimalarial activity was screened using an in vitro semi-quantitative test based on the method described by Deharo et al.13 In this procedure, 50 μL of freshly dissolved hemin chloride (0.5 mg/mL) in DMSO and 100 μL of sodium acetate buffer (0.5 M, pH 4.4) were combined with 50 μL of the test solution or control in a 96-well plate. The order of solution addition was crucial for accurate results. Then it was left for 18-24 hrs at 37 ºC. The plate was centrifuged at 4000 rpm for 10 minutes to separate the supernatant from the reaction mixture. The pH was measured after removing the supernatant, aiming for a range of 5.0 to 5.2. Each well was then washed with 200 μL of DMSO to remove unreacted hemin. After a second centrifugation and removal of the supernatant, the hematin was solubilized in 200 μL of 0.1 M NaOH to produce alkaline hematin, which was spectrophotometrically analyzed. The absorbance of the alkaline hematin was read at 405 nm using an ELISA reader. Ultrapure water served as the negative control, and chloroquine (CQ) dissolved in ultrapure water was used as the positive control. Results of the semi-quantitative tests (Figure 1–3) are presented as column diagrams, the average is the mean of 16 experiments (readings) and the error bars on the top of these columns represent the STDEV of the 16 readings.
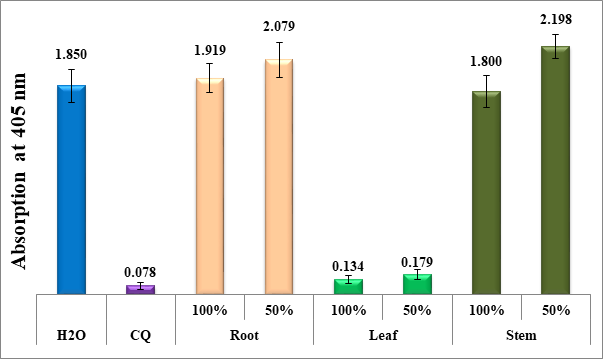
Figure 1 Column chart depicting the antimalarial effectiveness (measured by the absorption of dissolved beta-hematin at 405 nm) of aqueous extracts from the leaves, roots, and stems of dwarf nettle, compared to negative (water) and positive (CQ-0.1 mg/ml) controls. Each value represents an average of 16 separate experiments.
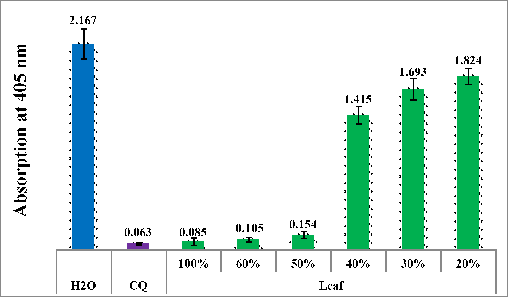
Figure 2 Column chart illustrating the antimalarial activity (measured by the absorption of dissolved beta-hematin at 405 nm) of aqueous extracts from the leaves of dwarf nettle, compared to negative (water) and positive (CQ-0.1 mg/ml) controls. Each data point represents the average of 16 separate experiments.
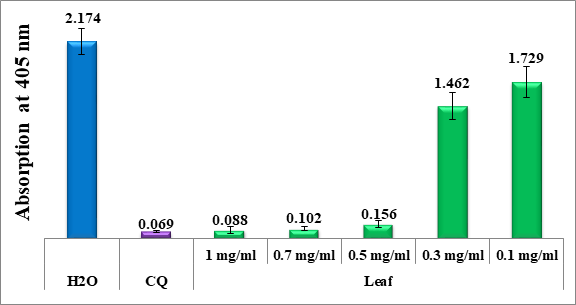
Figure 3 Column chart showing the antimalarial effectiveness (measured by the absorption of dissolved beta-hematin at 405 nm) of lyophilized leaf extracts of dwarf nettle, compared to negative (water) and positive (CQ-0.1 mg/ml) controls. Each data point reflects the average of 16 separate experiments.
Chromatographic analysis
The HPLC analytical experiments of the crude water extracts were run on ODS column of Waters (XBridge, 4.6 ID x 150 mm, 5 μm) with a guard column of Xbridge ODS, 20 mm x 4.6mm ID, 5 μm. The HPLC chromatographic gradient employs a mobile phase composed of 0.5% acetic acid (A) and acetonitrile (B), with a flow rate of 1 mL/min. The gradient begins with 100% A and 0% B. By 40 minutes, the mobile phase reached 70% A and 30% B. At 60 minutes, it further shifts to 40% A and 60% B. At 62 minutes, the mixture is 10% A and 90% B, maintaining this composition for 6 minutes. Following this period, the gradient shifts to 100% B and 0% A for 6 minutes. The HPLC system was equilibrated for 5 minutes with the initial acidic water mobile phase (100 % A) before injecting the next sample. All the samples were filtered with a 0.45μm PTFE filter. The PDA wavelengths range was from 210-500 nm. The flow rate was 1 ml/min. Injection volume was 20 μl and the column temperature were set at 25◦C. The HPLC system was then equilibrated for 5 minutes with the initial mobile phase composition prior injecting the next sample. The UHPLC chromatographic separations were performed on a Kinetex™ (Phenomenex, Torrance, CA, USA) column (C8, 2.6 µm particle size, 100 Å pore size, 100 x 2.1 mm), protected by a UHPLC SecurityGuard™ (Phenomenex, Torrance, CA, USA) cartridge (C8, for 2.1 mm ID column). The injection volume was 1 μL, the oven temperature was maintained at 35°C. The chromatographic separation was achieved using the same HPLC linear gradient program using formic acid instead of acetic acid at a constant flow rate of 0.4 mL/min over a total run time of 70 min. The samples were detected by a TSQ Quantum Access Max mass spectrometer in positive and negative ion mode using Electron Spray ionization (ESi) and full scan acquisition between the range of 100-1200 Da. Air was produced (SF 2 FF compressor, Atlas Copco, Belgium). Purified nitrogen was used as source and exhaust gases.
Figure 1 presents a column diagram illustrating the antimalarial efficiency of aqueous extracts from different parts of dwarf nettle (Urtica urens), namely leaves, roots, and stems by measuring the absorption of dissolved beta-hematin at 405 nm. This absorption is indicative of the extracts' ability to inhibit beta-hematin formation, a key target in malaria treatment.
The antimalarial efficacy of the dwarf nettle extracts is compared against two controls: a negative control (water) and a positive control (CQ-0.1 mg/ml, where CQ stands for chloroquineantimalarial drug). The negative control represents the baseline measurement of beta-hematin absorption in the absence of any treatment, while the positive control serves as a benchmark for effective antimalarial activity. According to the column diagram in Figure 1, the extracts from the roots and stems of dwarf nettle show no significant antimalarial activity, while the leaf extracts exhibit potential, at 100% and 50%. Phenols and flavonoids, crucial phytochemicals, differ notably between the leaves and roots of the Urtica urens plant. Typically, leaves have higher levels of flavonoids, which help protect the plant from UV radiation, pathogens, and environmental stress. These compounds are mainly concentrated in the outer layers of the leaves and play a key role in pigmentation and antioxidant defense, making them more effective in inhibiting beta-hematin.
Figure 2 demonstrates the effectiveness of the active leaf extract of dwarf nettle when diluted from 100% to 20%. The results indicate that the leaf extracts retain their antimalarial activity up to a 50% concentration. However, once diluted to 40%, the antimalarial effect disappears. This decline in efficacy at lower concentrations could be due to a reduced concentration of the active compounds responsible for antimalarial activity. As the extract is diluted, the concentration of these bioactive compounds decreases, potentially falling below the threshold needed to inhibit the beta-hematin effectively.
Figure 3 indicates that the leaf extract remains effective at concentrations between 1 mg/ml and 0.5 mg/ml, but becomes inactive at a concentration of 0.3 mg/ml. The decrease in activity at lower concentrations could be attributed to the reduced presence of the active compounds responsible for the antimalarial effect. As the concentration drops below 0.5 mg/ml, the amount of these compounds may no longer be sufficient to inhibit the beta-hematin effectively, leading to a loss of activity at 0.3 mg/ml.
LC-MS analysis of water of dwarf nettle extracts
The bioactive components of the dwarf nettle leaf extract were separated and analyzed using the UHPLC-PDA-ESi-MS system, as depicted in Figure 4. Mass spectrometry identified specific flavonoids (flavanols and flavanones) in the active water fraction of the leaf extract, detailed in Table 1.
|
# |
Name |
*RT (mins) |
m/z [M-H]- |
m/z [M+H]+ |
|
1 |
Myricetin 3-O-rutinoside (flavonol) |
53.13 |
625 |
|
|
2 |
Isorhamnetin 3-O-glucoside 7-O-rhamnoside (flavonol) |
53.5 |
625 |
|
|
3 |
Kaempferol 3,7-diglucoside (flavonol) |
54.02 |
610 |
|
|
4 |
Kaempferol-3-O-rutinoside (flavonol) |
54.61 |
595 |
|
|
5 |
Rutin (flavonol) |
55.56 |
609 |
|
|
6 |
Apigenin 7-O-diglucuronide (flavonol) |
59.27 |
622 |
|
|
7 |
Kaempferol 3-O-(6''-acetyl-galactoside) 7-O-rhamnoside (flavonol) |
61.12 |
636 |
|
|
8 |
Luteolin 7-O-diglucuronide (flavone) |
76.34 |
639 |
Table 1 Flavonoids found in Urtica urens water infusion with their MS spectral characteristics.
*RT: retention time
Furthermore, Figure 5 shows the chemical structures of these identified active ingredients. By examining these structures and their key functionalities such as hydroxyl groups, ketones, etc., one can better understand how they may interact with heme, elucidating their roles and enhancing their efficacy, which supports the assessment of their potential therapeutic benefits against malaria. Flavonols and flavones are types of flavonoids, which are a diverse group of phytonutrients found in fruits, vegetables, and plant-based natural products. They have several important functions and health benefits particularly as antimalarial effect.5–10
Flavonoids, with their varied chemical characteristics, can interact with heme through chelation or binding, potentially disrupting the conversion of free heme into hemozoin, which is a detoxification process. The hydroxyl groups present in flavonoids can function as Lewis bases, binding to the iron within the heme molecule and forming a stable complex. This interaction might prevent the heme from polymerizing into hemozoin. Additionally, flavonoids could interfere with the enzymes responsible for hemozoin formation or alter the pH within the parasite's food vacuole, further affecting polymerization. Some flavonoids might also induce oxidative stress by generating reactive oxygen species (ROS), which can exacerbate damage from free heme and lead to the parasite's death. To better understand these interactions and their stability, molecular modeling and docking studies are crucial in determining how readily these complexes form.5–8
This study demonstrates that aqueous extracts from dwarf nettle (Urtica urens), particularly those derived from the leaves, possess significant antimalarial activity by effectively inhibiting beta-hematin formation. While the extracts from roots and stems showed minimal activity, the leaf extracts exhibited notable potential, maintaining efficacy up to a concentration of 50% and proving effective at concentrations between 1 mg/ml and 0.5 mg/ml. The analysis revealed that the potential antimalarial properties of these leaf extracts are largely due to their flavonoid content. These flavonoids have demonstrated antiplasmodial effects, suggesting that they interact with heme in a way that inhibits malaria parasite growth. The study underscores the promise of these flavonoid-rich extracts as viable candidates for malaria treatment. Furthermore, the accessibility, affordability, potency, safety, and ease of preparation of these water extracts enhance their potential as a practical remedy for malaria. These findings pave the way for the development of new, effective antimalarial drugs derived from natural sources, offering innovative approaches to combating this pervasive disease. So far, the scientific literature fully confirms the absence of toxicity and resistance in humans. Consequently, future research will prioritize identification and chemical characterization of possible active ingredients .Further fractionation and purification of the crude extracts as well as in vivo testing need to be done. We hope this will assist in guiding future efforts and identifying areas for further exploration
None.
The authors declare that there is no conflict of interest.
None.

©2024 Abu-Lafi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.