eISSN: 2379-6367


Research Article Volume 9 Issue 5
1School of Science & Technology, Ana G. Mendez University, USA
2Department of Chemistry, College of Liberal Arts and Sciences, University of Illinois at Chicago, USA
3Department of Pharmacology and Toxicology, School of Medicine, University of Puerto Rico, USA
Correspondence: Beatriz Zayas, PhD. School of Science & Technology, Ana G. Mendez University, PO Box 21150, San Juan, 00928, PR
Received: October 19, 2021 | Published: October 29, 2021
Citation: Carrasquillo G, Pinet-Velez N, Velez C, et al. Cell death induction of dibutyl phthalate (DBP) on primary brain cells from adult zebrafish. Pharm Pharmacol Int J. 2021;9(5):219-225. DOI: 10.15406/ppij.2021.09.00348
The presence of phthalates as plasticizer on pharmaceutical products as well as in aquatic environments requires serious attention. Phthalates are compounds widely used in the manufacturing industry and has been associate with multiple adverse health effects to human. Among the family of phthalates Dibutyl phthalate (DBP) is well used for pharmaceutical products. Limited information however has been generated on the effect of DBP on the brain. The objective of this investigation was to characterize potential neurotoxicity resulting from short term exposure to DBP. Considering the efficacy and sensitivity of the zebrafish model as an early stage- screening tool, and their neurological and biochemical similarities to those of human, this study evaluates the effect of DBP on primary brain cells of the adult zebrafish. This well optimized and implemented methodology can be consider a guide for future studies using primary cells of adult zebrafish given the reduction on reagents, and the number of cells used for treatment. For indirect confirmation of extracted nervous tissue, the Acetylcholinesterase activity (AChEs) assay kit was implemented. In our study primary cells from adult zebrafish brain were exposed to DBP for 24 hours at aerobic environment at concentrations from 3uM to 100μM. The effect on cell viability as well as cell death mechanisms was determined. Results indicate that adult primary brain cells viability is compromised as low as 8uM of DBP. In terms of mechanism, cells undergo apoptosis and autophagy at concentrations of 8μM and 100μM. This study confirms that low concentrations of DBP over a 24-hour period induces apoptosis and autophagy, potentially activating degradation of brain cells and altering biochemical processes of the nervous system. This research presents a novel approach for future molecular studies using brain primary cells as model to screen pharmaceuticals and environmental agents for risk assessment.
Keywords: phthalates, dibutyl phthalate (DBP), pharmaceuticals, zebrafish, primary brain cells, neurotoxicity, apoptosis, autophagy, environmental risk assessment
Dibutyl phthalate (di-n-butyl phthalate, DBP) is used extensively as a plasticizer, producing flexible plastics found in a variety of consumer products, including medications, beauty and personal care products, paints, and other household solvents.1,2,3 DBP has also been identified as inactive ingredients (excipient) in multiple currently used medication recommended for heart conditions; high blood pressure; Crohn’s disease; inflammatory bowel disease; ulcerative colitis among others.4 The presence of DBP as well as other plasticizers in many aspects of our daily activities is alarming and requires total understanding of their potential health damage.
DBP is the diester obtained by the formal condensation of the carboxy groups of phthalic acid with two molecules of butan-1-ol. In terms of its physical properties, its highly lipophilic, increasing its capacity to accumulate in adipose tissue and its retention. Also, since it is a liquid, it can easily penetrate the soil and contaminate groundwater and nearby streams. Due to non-chemical inclusion, plasticizers such as DBP and DEHP leach out of plastic and have been found in body fluids such as in amniotic fluid, breast milk, urine, and serum.5 It appears to have relatively low acute (short-term), but chronic (long-term) toxicity.6 Among the identified health effects, recent studies show that DBP has toxic effects in fetal development, endocrine disruptor/damage, reproductive toxicity, testicular and ovarian toxicity, respiratory distress, and nervous system toxicity.6-9 Recently, EPA proposed the designation of Dibutyl Phthalate (CASRN 84-74-2) as High-Priority Substance for Risk Evaluation in August 2019.10-12 The continued use and plastic production by global plasticizer market including the pharmaceutical industry does not seem to be changing in the near future and Phthalates as an Environmental Contaminant will continue to increase in comparison to non-phthalate products.13-15
The Federal Drug Administration also has been concerned with the presence of plasticizers as excipients in commonly used medication and in 2012 FDA announced the availability of a guidance for industry entitled ‘‘Limiting the Use of Certain Phthalates as Excipients in CDER- Regulated Products’’.5 This guidance provides the pharmaceutical industry with CDER’s current thinking on the potential human health risks associated with exposure to DBP and DEHP.
This research applies adult zebrafish brain tissue as a model to determine cellular events resulting from exposure to phthalates at levels potentially found in the environment.16,17 Applications of Zebrafish, as a neurotoxicological model, have been reported with high sensitivity for the analysis of novel compounds as well as a screening model for detecting toxicity and drug efficacy.6 As model to determine toxicity, zebrafish has strong resemblance to human making it an ideal model for developmental toxicity as well as similarities with the nervous system6-8 making it an applicable model for health risk assessment of drugs or contaminants. The zebrafish have basic nervous systems organization in comparison with other vertebrates; many neural circuits and cell types relevant to human studies are in zebrafish. Many genes implicated in the human neurological nervous system are in zebrafish as well. The zebrafish embryonic model has been used to study vertebrate development for many years.8 Our study, however, will implement the adult stage zebrafish. This will resemble even closer to a developed adult human or aquatic species in continuous exposure to low concentrations of DBP in a daily base.
This study adds an additional significant methodological contribution by applying a zebrafish primary cell model, from adult brain tissue as an optimized methodology to previous references based on transformed brain cells.18 This optimized methodology presents the advantages of being more representative of “in-vivo” toxic effects of environmental contaminants over aquatic species.
This study demonstrates that even at low concentrations of DBP, cell death related events such as apoptosis and autophagy can be observed after 24 hours of continuous exposure. This research presents a great opportunity to develop future molecular studies using primary (not transformed) cell models applied for environmental and pharmaceutical risk assessment.
Institutional ACUC and biosafety compliance
The reported study was evaluated following Animal Care and Use Committee (ACUC) federal regulations and approved by the Ana G Mendez ACUC & Biosafety Committee with the following approved protocol numbers: IACUC #A1-016-18; IBC #B01-056-18. The zebrafish used were handled as recommended by the American Veterinary Medical Association Guidelines for the Euthanasia of Animals: 2013 Edition. Similar guidelines are incorporated in the ARRIVE guidelines.19,20
Zebrafish population and care
Approximately 60 to 65 adult zebrafish were used in this study. To reduce experimental variability and similarities among the zebrafish population a local and ecologically maintained fishery (Caribe Fisheries, Lajas, Puerto Rico, 00667) was used as the source of adult zebrafish. Once the zebrafish arrive at the research laboratory, are located on a fish water tank with appropriate acclimated water for at least 12 hours prior to used. During that time the area for the animal procedures (euthanasia, decapitation, and dissection of the brain) is prepared. As instructed the preparations for euthanasia of finfish should be very similar to the preparations for anesthesia. Pain and distress applied in this study falls within classification C since euthanasia is performed in accordance with the most recent AVMA Panel and the CITI program module certification for studies using fish. Implemented euthanasia procedures produce rapid unconsciousness and subsequent humane death.19,20
Dissection method
Primary cells from the nervous system of Zebrafish were acquired following humane handling of the fish for decapitation and extraction of the brain tissue. Dissection and culture of adult zebrafish brain cells required multiple adaptations from reference21 as summarized here:
Primary cells dissociation
Single cell dissociation of adult zebrafish brain: Applying a biological safety cabinet sterile condition centrifuge brain tissue (5 minutes) and extract the condition medium by gently pipetting with a 5ml pipet, without touching brain tissue. Add 5ml of washing solution to rinse the rest of the condition medium. Centrifuge and remove the washing solution again with a 5ml pipet without touching the tissue. Add 3 ml of digestion solution and incubate the brain tissue at 37°C for 10 min. After incubation, gently dissociate the brain tissue by slowly pipetting up and down 10 times with a 10ml pipette; Incubate brain tissue again at 28°C for 10 minutes and then pipet up and down 10 to 13 times with 5ml pipette; Add 2ml of PBS to remove the digest solution and centrifuge the cell suspension. Carefully decant the supernatant and resuspend the pellet in the washing solution. Pipet up and down with a 1,000µl micropipette. At this stage, verify under the microscope that a single cell suspension has been obtained. Use a syringe (19Gx1 ½ "- 1.1x38mm) to break up residual brain tissue if needed; Centrifuge the cell suspension again, remove the supernatant and resuspend the pellet with 1ml of conditioned medium. Cultures are incubated at 28°C for 24 hours before treating or exposing for toxicity assays.
After dissection and primary cells dissociated it was important to confirm cells were morphologically intact and healthy. To evaluate the morphology of the primary brain cells of adult zebra fish in cultures, images were obtained with an Olympus CKX41 light microscope (100 X) followed by fluorescence microscopy. Fluorescence microcopy was implemented by preparing a 100ul of cellular suspension in 900ml of PBS initially stained with Cell Brite Orange 30022 to see the membrane and then with Hoechst 333442 to identify the nucleus. The resulting image was captured with an Olympus FSX100 fluorescence microscope in 40x blue and red filters in which the healthy orange membrane and the blue nucleus can be identified.
Acetylcholinesterase (AChEs) activity assay
As a way of confirming we had extracted mainly nervous tissue, the Acetylcholinesterase activity (AchEs activity) assay kit was implemented (Sigma/Aldrich, MO). Acetylcholinesterases (AChEs) are enzymes that hydrolyze the neurotransmitter acetylcholine (ACh) to acetate and choline. It is also found in membranes of red blood cells, motor and sensory fibers, nerves, and central and peripheral nervous tissues. This assay is here applied to determine the activity of acetylcholinesterase in brain tissue homogenates. Tissues adjacent to the brain (eyes) were isolated as control to compare the AchEs activity expected of brain tissue. The assay determines the activity of acetylcholinesterase in brain tissue homogenates. The AChEs activation analysis compared the brain tissue of adult zebrafish with other zebrafish tissues (eyes) and with secondary cells of colorectal cancer as a negative control. The protocol for determination of the AChEs activity in the primary brain cells from adult zebrafish briefly: i. An experimental plate with a minimum of 10,000 primary cell per well was prepared and 100µl of working reagent was added. Each plate included isolated cells, negative control, and calibrator; ii. Experimental plate is incubated at room temperature x 30 minutes; iii. Acetylcholinesterase Activity is analyzed with a Fluorostar OMEGA (BMG labtech, Cary, NC) following manufacturer’s instructions.
DBP Vehicle determination
For determination of the appropriated vehicle for DBP stock solutions, Methanol & Ethanol were tested. The optimal solvent should induce limited if any, background toxicity as vehicle for DBP treatments. DMSO as vehicle for phthalates has been used by our research group in previous publication with human cells,18 however reviewed literature22 had indicated high toxicity of DMSO on zebrafish and therefore was not selected as solvent. To determine the least toxic solvent (vehicle) in zebrafish primary brain culture were treated with 10µl of Methanol or Ethanol on 200µl of cell suspension in medium and incubated for 24 hours at 28ºC with 5% of CO2. After 24 hours of exposure and for viability determination cells were stained with 10µL of Prestoblue reagent (Thermo Fisher) and incubated for 30 minutes at 37℃. Analysis of viability was determined by the fluorimetry (indicative of cell viability) Fluorostar OMEGA (BMG labtech, Cary, NC) with the 544/590nm filters.
Cell growth inhibition
Once the appropriate vehicle for DBP with minimum toxicity on primary zebrafish brain cells was identified, growth inhibition of DBP on primary brain cells was assess. Aliquots of DBP dissolved Ethanol were prepared 24-48 hours prior to exposure. To determine growth inhibition, primary cells were seeded (approximately 10,000 cells/well) on 96 well plate with a final volumen of 200 µL in each well (BRAND plates cell Grade, Sigma Aldrich, MO). Cells were exposed to DBP and treated for 24 hours at concentration of 3µM, 6µM, 8µM, 15µM, 20µM, 75µM and 100µM of DBP (in triplicates). After 24 hours of exposure and for viability determination cells were stained with 10µL of Prestoblue reagent (Thermo Fisher) and incubated for 30 minutes at 37℃. Analysis of viability was determined by the fluorescence generated (indicative of cell viability) Fluorostar OMEGA (BMG labtech, Cary, NC) with the 544/590nm filters.
Apoptosis cell death effect of DBP
For determination of apoptosis induction of DBP as part of the cell death mechanism in primary brain cells of adult zebrafish the Annexin V apoptosis assay was implemented. The annexin V assay is used as an indicator of the early stages of apoptosis. This assay allows for detection of phosphatidylserine (PS) migration to the exterior surface of cells, a hallmark in apoptotic cells. Experimental plate with primary cell (10,000 x 100ul) was prepared and DBP added in concentrations of 8uM and 100uM for 24 hours. Samples included vehicle (without DBP), positive control and treatments incubated for 24 hours at 28C and 5% CO2. Cells were stained with the FITC functionalized annexin V conjugate, Hoechst DNA stain and propidium iodide to screen for necrosis (Biotium, Hayward, CA). Samples were analyzed using the Nucleo Counter NC3000 system (Chemometec, Allerød, Denmark). A one-way ANOVA was performed to determine statistically significant results.
Autophagy cell death effect of DBP
For determination of autophagy as cell death in primary brain cells of adult zebrafish the monodansylcadaverine (MDC) from Sigma Aldrich (Saint Louis, MO) was implemented following manufacturers recommendation. Briefly experimental plate with primary cells (100ul) was prepared and DBP added in concentrations 8uM and 100uM. Vehicle control and positive controls were included. Samples incubated for 24 hours at 28C and 5% CO2. After 24 hours of exposure to DBP autophagy induction is determined by adding 1.67ul of MDC (monodansylcadaverine; autophagy indicator) and incubate for 30 minutes at 28C and 5% CO2. Samples were analyzed using the Nucleo Counter NC3000 system (Chemometec, Allerød, Denmark). A one-way ANOVA was performed to determine statistically significant results.
This research main goal has been to characterize the cellular events that resulted from exposure to dibutyl phthalate (DBP) as an environmental contaminant given its detection on aquatic environments.2 Cellular events were assessed using primary brain cells from adult zebrafish since it has been identified as a neurotoxicological model for human exposure.6,7 Zebrafish has basic nervous systems organization in comparison with other vertebrates; many neural circuits and cell types relevant to human studies are in zebrafish. Many genes implicated in the human neurological nervous system are in zebrafish as well, suggesting that the biochemical events could be similar in humans and zebrafish and perhaps the toxic and negative effects as result of contaminants exposure.
The brain dissection protocol has been highly optimized as part of this research integrating reported methodologies. As summarized in Figure 1, the head dissection protocols include multiple steps: pre-dissection, dissection, and post-dissection. The pre-dissection describes key solutions and settings necessary to eliminate potential contaminations as well as a control environment for consistent dissection process. The post-dissection includes the preparation and expansion of primary brain cells from dissected brain prior to treatments with DBP as well as cleaning and waste handling. Once primary brain cell cultures were established toxicity assays were implemented at DBP concentration from 3uM to 100uM to determine the effects in cellular viability (toxicity), apoptosis and autophagy as described above. Images from the dissection and primary cells cultures in Figure 1 confirms a stable and healthy cell population. Hoechst 333442 stained cells as presented in Figure 1 shows cells with intact nucleus and membranes.
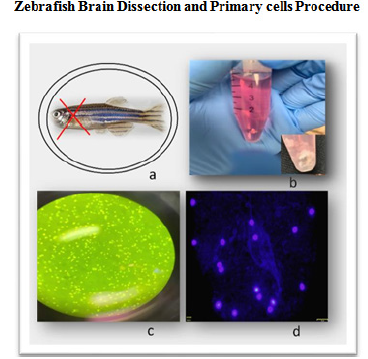
Figure 1 Adult zebrafish brain dissection and primary cell culture procedure: a. Zebrafish head dissection region; b. Isolated brain tissue from adult zebrafish in neural culture medium; c. Light microscopy image of zebrafish primary brain cells in culture; d. Fluorecesence microscopy image of primary brain cells with Olympus FSX100 fluorescence microscope (40x) blue and red filters. Cells are stained with Hoechst 333442 for nucleus identification in blue.
Acetylcholinesterase (AChEs) activity assay
The activity of the enzyme AChEs is used as a biomarker to confirm the presence of neuronal cells. This was performed by using the acetylcholinesterase activity assay kit from Sigma/Aldrich and analyzed on a Fluorostar Omega fluorometer/spectrophotometer from BMG. The AChEs activation analysis compared the brain tissue of adult zebrafish with other zebrafish tissues (eyes) and with secondary cells of colorectal cancer. The results of these experiments indicate that dissected primary brain cells have more AChEs activity than eyes tissue and colorectal cancer secondary cells. A one-way ANOVA and multiple comparison were performed, with statistically significant differences of brain cells in the ACheEs activity (P<0.0001) in comparison to the negative controls. The results clearly confirm that the dissected tissue was representative of neurological tissue. Figure 2 presents acetylcholinesterase (AChEs) activity of primary brain cells of the adult zebrafish in contrast with also dissected zebrafish eyes and human Colo-205 cancer cells.
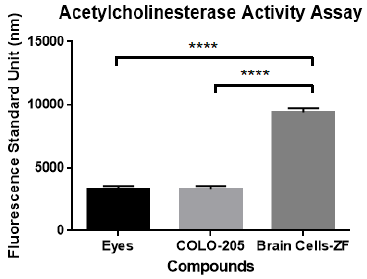
Figure 2 Acetylcholinesterase (AChEs) activity of primary brain cells of adult zebrafish in contrast with dissected adult zebrafish eyes and human Colo-205 cells. A one-way ANOVA and multiple comparison analysis demonstrated statistically significant difference in the AChEs activity (P <0.0001) of zebrafish brain primary cells in comparison to controls.
DBP vehicle determination
Prior to exposing dissected brain cells of adult zebrafish to DBP, the appropriate solvent as vehicle for the treatment as well as negative control was determined. The optimal selected solvent should induce limited if any, background toxicity as vehicle for DBP treatments. For the determination of the appropriate vehicle, primary brain cells dissected from adult zebrafish were treated to Methanol and Ethanol both solvents for 24 hours were tested at two percentages (0.05 and 0.1%). Although both demonstrated limited toxicity, Ethanol demonstrated to be less toxic in comparison to methanol. Figure 3 demonstrated more live cells in ethanol in comparation with cells treated with methanol. The results were consistent with reported studies.20
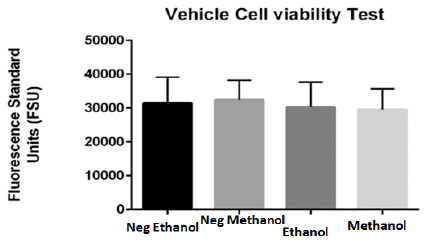
Figure 3 Viability effect of tested vehicles (ethanol and methanol) on primary brain cells of adult zebrafish for 24 hours. Blank corrected results indicate no significant difference between cells in culture media without tested vehicle and cells treated with 0.05% vehicles (ethanol and methanol). Both tested solvents presented minimum toxicity, however Ethanol demonstrated to be less toxic in comparison to Methanol and was selected as vehicle.
Cell growth inhibition
After identifying the appropriate vehicle for DBP stock solutions and having an established culture of primary brain live cells toxicity of DBP on brain cells was determined. Primary brain cell cultures of adult zebra fish were treated for 24 hours at concentration of 3µM to 100µM of DBP. The results showed significant changes in viability percentage at 75µM and 100µM. Other studies, however, have reported effect in viability at lower concentrations of DBP on aquatic organism.16 Figure 4 demonstrate that at 8µM no significant effect in viability was found at a 24hours of in- vitro treatment, however. At 75µM and 100µM clear decrease in viability was observed.
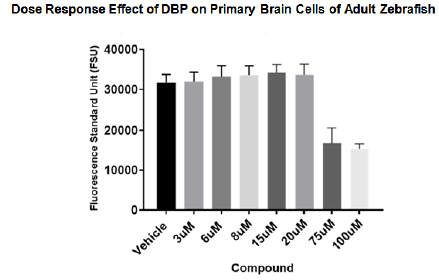
Figure 4 Growth inhibition effect of DBP on primary brain cells of adult zebrafish at 24 hours. Primary brain cells of adult zebra fish were treated in culture for 24 hours at concentration of 3µM to 100µM of DBP. Blank corrected analysis showed significant changes in viability percentage at 75µM and 100µM.
Apoptosis cell death effect of DBP on primary brain cells from adult zebrafish
Recent studies show that DBP is toxic and has apoptotic effects on mouse neural cells at low concentrations and that effect has been seen with exposures from three hours to 40 hours.23 After observing in our study that DBP can induce cell viability inhibition on zebrafish primary brain cells it was necessary to determine the mechanisms involved. The apoptosis cell death is a type of death that includes programmed changes in cellular morphology that normally occurs in organisms to maintain the balance between living and dead cells, but it can also be chemically induced.19 The Annexin V-FITC Apoptosis detection assay from Sigma was used to determine if apoptosis was occurring in the treated primary brain cell of adult zebrafish and analyzing it on a Fluorostar Omega fluorometer/spectrophotometer from BMG. As presented in Figure 5, the results of these experiments clearly indicates that primary brain cells can undergo apoptosis after 24 hours of treatment at concentrations of 8μM and 100μM. A one-way ANOVA and multiple comparison were performed, to determine statistically significant differences in the apoptotic effect (P<0.05) in comparison to negative controls.
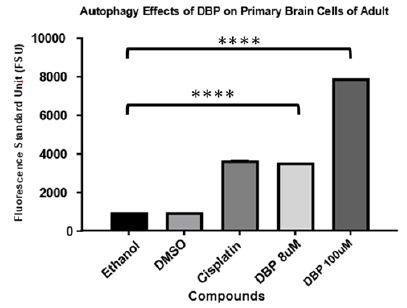
Figure 5 Apoptosis effect of DBP on primary brain cells of adult zebrafish for 24 hours. Cisplatin and DMSO (vehicle) were used as positive and negative control respectively. The blank corrected analysis demonstrated apoptosis induction of DBP at 8µM and 10µM after a one-way ANOVA and multiple comparison with significant difference of p<0.001) in comparison to the negative control.
Autophagy cell death effect of DBP on primary brain cells from adult zebrafish
The autophagy cell death is a type of degradation that occurs from the inside out in cells.20 Recent studies reported that exposure to dibutyl phthalate causes autophagy like stress response in cells.21 To assess similar effects on primary brain cells of adult zebrafish our study implemented the monodansylcadaverine (MDC) assay as an autophagy indicator after exposure to DBP for 24 hours. MDC is an auto-fluorescent compound that has been shown to accumulate in acidic autophagic vacuoles. The concentration of MDC in autophagic vacuole is the consequence of an ion-trapping mechanism and an interaction with lipids in autophagic vacuoles (autophagic vacuoles are rich in membrane lipids).20 The use of MDC staining is a rapid and convenient approach to assay autophagy. Results were read on a Fluorostar Omega spectrophotometer from BMG. As presented in Figure 6 results indicated that cells undergo autophagy after exposure for 24hours to DBP at 8μM and 100μM. A one-way ANOVA and multiple comparison were performed, and the experimental samples presented statistically significant differences indicative of autophagy induction (P<0.001) in comparison to the negative controls.

Figure 6 Autophagy effect of DBP exposure on primary brain cells of adult zebrafish for 24 hours. Cisplatin and DMSO (vehicle) are used as positive and negative control. A one-way ANOVA and multiple comparison were performed demonstrating DBP treated cultures presented statistically significant difference for autophagy induction (P<0.0001) in comparison to the negative controls.
The main objective of this research was to characterize cellular events resulting from exposure to dibutyl phthalate a plasticizer found in pharmaceuticals and aquatic environments, applying primary brain cells of the adult zebrafish. The implemented methodology can be considered a guide for future studies using primary cells from the nervous system of adult zebrafish. The improved and advanced of methodology resulting from this research represents an optimization to previous references and studies based on primary brain cells. The optimization includes reduction of solvents, medium, reagents and even the number of cells used for treatment and exposure. This is an advantage that can reduce not only chemical reagents but number of animals as well.
The findings of this in-vitro study can be consider representative of potential in-vivo effects. Given the presence of phthalates in multiple human care products as well as in aquatic environments a continues exposure can be expected. This continues exposure brings concern not only on bioaccumulation but also on the capacity of such molecules to cross the blood brain barrier (BBB) and therefore affecting brain cells and the nervous system. The applied zebrafish model has been implemented by other researchers to demonstrate the permeability and distribution of fluorescent dyes and drugs across the BBB.22 The study however did not elaborate on phthalates but included molecules with structural similarities. Other studies however do present reasons to believe that phthalates can cross the BBB in rats. The study clearly states that exposure to Di-(2- ethylhexyl) Phthalate during perinatal period impairs the dendritic growth of pyramidal neurons in rat offspring.23,24
The value of implementing primary cells from adult organism relies on their representation of organisms in real environments that can be affected by continuous exposure to environmental contaminants in this modern and industrialized world. The findings of this investigation indicate that DBP at low concentrations can induce damage to brain cells of adult zebrafish in a dose dependent manner. It is important to state that the implemented AChEs Activity Assay to confirm dissected brain cells can be consider as an indirect confirmation of the cell’s origin. This might be seen as a limitation, if heterogenous cells were used. It is however from our perspective a more representative scenario of brain tissue and surrounding cells.
Results demonstrated that at 75uM and 100uM brain cell viability was compromised. In terms of the specific mechanism of cell death, our results indicated that 24-hours exposure to DBP can induce apoptosis and autophagy at concentrations of 8μM and 100μM of DBP. The fact that a decrease in viability is not observed at 8uM but apoptosis was observed, can be explained by understanding that early apoptosis can be initiated at 8uM, however a continues exposure to low concentrations can trigger a significant cell death effect. These findings generate high concern of how humans as well as aquatic species can be affected by longer and continues exposure to low-concentrations of DBP as well as other phthalates and emerging contaminants.
In terms of pharmaceuticals and medications, DBP is commonly used as excipients on prescription (RX) and nonprescription over the counter (OTC) medicinal products and dietary supplements in the United States and Canada since 1995. The permitted amount used will vary depending on its usage and manufacturing regulation. The maximum amount of DBP used in specific FDA-approved drug products ranges from 1.70 mg for a delayed-action, enteric-coated tablet formulation to 11.18 mg for an extended-release capsule product. Also, DEP was approved by FDA in 2010 at amounts ranging from 0.5 mg for an uncoated, chewable tablet to 16.8 mg for a delayed-action, enteric-coated capsule.21 Recent accepted levels can be changing. This is relevant since it is not really known to what level of DBP we are exposed considering all existing sources.
This study presents evidence of specific cellular events triggering cell death on the nervous system of zebrafish. It is important to add that both detected cell death processes, apoptosis and autophagy, can result in degradation of brain cells and significantly adverse effects on natural biochemical processes of the nervous system of human and aquatic species. Recent studies suggest that exposure DEHP, a highly used phthalate, induces apoptosis and autophagy in human lymphoblast cells but also resulted on degradation of key organelles such as mitochondria.16,18,25-27
In conclusion our study clearly demonstrates that exposure to dibutyl phthalate at low and high concentrations as potentially found in the environments or a healthy product induces brain cell death. It also demonstrates that the use of primary cells provides more relevance to this type of risk assessment study, recognizing that each organism, in this case, each zebrafish brain dissected, represents individual organisms that can resemble human variability and sensitivity as well.
The authors acknowledge the financial support from the PR-CEN National Science Foundation grant award# 1736019 and the National Institute of Health grant No. 5P20GM103475-18 provided to the PR-INBRE. We also want to thank Dr. Maria Ortiz from UAGM, Cupey for her emotional support and guidance; the undergraduate student Alejandra Rivera and research associate Karoline Rios from the ChEMTox Lab for their help and support.
Authors declare that there is no conflict of interest.

©2021 Carrasquillo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.