eISSN: 2379-6367


Review Article Volume 8 Issue 4
1Escuela de Medicina Veterinaria y Zootecnia, Universidad Pedagógica y Tecnológica de Colombia, Lead Researcher, Grupo de Investigación en Bioquímica y Nutrición Animal GIBNA, Colombia
2Universidad Pedagógica y Tecnológica de Colombia, Researcher, Grupo de Investigación en Bioquímica y Nutrición Animal GIBNA, Colombia
Correspondence: Carlos-Eduardo Rodríguez, Escuela de Medicina Veterinaria y Zootecnia, Universidad Pedagógica y Tecnológica de Colombia, Lead Researcher, Grupo de Investigación en Bioquímica y Nutrición Animal GIBNA, Tunja, Boyaca, Colombia, Tel +573044150615
Received: July 14, 2020 | Published: July 30, 2020
Citation: Rodriguez-Molano CE, Torres SU, Monrroy LEN. Background on the control of the cattle tick R. (B.) microplus and the use of coumarin substances as an alternative. Pharm Pharmacol Int J. 2020;8(4):215-232. DOI: 10.15406/ppij.2020.08.00299
Rhicephalus (Boophilus) microplus (R. (B.) microplus) is a hematophagous ectoparasite of Indo-Asian origin that is found in tropical and subtropical regions, which has expanded its geographical distribution as a result of climate change, migrating to northern latitudes and higher altitudes. This species creates estimated economic losses between $13.9 and 18.7 million dollars per year, generating direct and indirect effects on livestock such as low productivity and production rates, decreased reproduction, and even death through the transmission of diseases associated with this species, including diseases known as TBD (tick borne diseases), which are a public health problem in countries with high rates of occurrence, such as the United States, the United Kingdom, Russia, France, Australia and Brazil.
The chemical control of cattle ticks began in 1895 with the use of arsenic baths that reduced infestation of this species in herds in Australia. Today, many formulations and techniques have been developed to control of this species; however, malpractice, underdosing and/or overuse of these substances have allowed this species to develop different types of resistance, which have documented worldwide. Countries with a high number of resistance reports include Mexico, Brazil and Australia.
These factors serve as a starting point for research that seeks to provide economically and environmentally viable alternatives for the control of cattle ticks, which make use of different types of plant extracts obtained from many species. As a result, high control rates at different stages of this species using various compounds with a less harmful effect on the environment have been achieved, such as with coumarins, which are obtained from chemical reactions using methodologies designed with the concept of green chemistry. This paper sought to provide an overview and approximation of the traditional control of R. (B.) microplus and control alternatives that use coumarin compounds.
Keywords: coumarins, control, resistance, acaricides, vector, environmentally viable alternatives
The cattle tick R. (B.) microplus is a hematophage ectoparasite of great importance worldwide because it causes considerable economic losses to the meat and dairy industry,1 losses that they are directly associated with a lower weight gain and milk production.2 In addition, it is a vector of zoonotic diseases, including anaplasmosis, babesiosis, ehrlichiosis and Lyme disease.3,4
Given the importance of this pest, chemically synthesized acaricides have traditionally been used to reduce infestations in cattle, generating a series of problems associated with environmental pollution, loss of food quality through the presence of agrochemicals in meat and milk, and significant economic losses worldwide.5,6 Different authors have reported that R. (B.) microplus has generated resistance to acaricides as a result of continuous and improper use,7 generating interest in investigating plant extracts with ixodicidal properties as a sustainable alternative with low or no toxicity to mammals, rapid degradation in the environment and greater impediment for the development of resistance that is economically viable.8,9 Studies have used different species, showing high levels of developmental inhibitory action for eggs and adult control, suggesting that they are a viable alternative for the control of this tick.9-11
Secondary metabolites such as isoflavones, flavones, flavonols, neoflavones and coumarins have been isolated from plant extracts.12
Coumarins have been studied since 1860, revealing wide range of antiviral, antiparasitic, antifungal, and insecticidal activities, among others.13 Because of their importance in different types of industries, there is a large amount of research on obtaining them, isolating them naturally or through chemical synthesis. As a result, there are approximately 1300 coumarins isolated from different plant families.14-16 The various procurement processes have generated variations in structure and possible use, so the insecticidal activity has been studied, demonstrating great potential for the control of different species of mosquitoes, ticks and other pests that transmit diseases in humans, animals and plant species.17
This paper was developed with a review of research literature and publications of scientific importance that have impacted knowledge on the cattle tick R. (B.) microplus, mainly through exploration of types of control with an emphasis on plant extracts containing coumarins. The cited articles were considered for this review, but not all studies on which the research was based were included.
The objective was to provide an overview and approximation of the traditional control of R. (B.) microplus and control alternatives that use coumarin compounds, taking into account the literature and discussions on the use of plant extracts for controlling this ectoparasite.
The cattle tick R. (B.) microplus is a species of Indo-Asian origin18 that is widely distributed in tropical and subtropical regions between 32°N and 35°S latitude, in areas with an average annual rainfall of 750 to 1000 mm, a temperature range between 12°C and 24°C and altitudes from 250 to 1600 meters above sea level;9,19 however, as a result of climate change, R. (B.) microplus has expanded its geographical distribution to northern latitudes and higher altitudes,20 as reported by Pulido-Herrera et al.,18 who observed the presence of R. (B.) microplus at altitudes above 2,750 m.a.s.l., with temperatures below 12°C and 500 mm of rainfall, in the Cundiboyacense highlands of Colombia.
This ectoparasite lives on the surface of a host,21 feeding on the blood without mortality but can transmit different pathogens.22 Its life cycle is divided into two phases: the non-parasitic phase comprised of pre-oviposition, oviposition, pre-hatching and hatching, and the parasitic phase with feeding, molting and mating. The duration of the cycle is influenced by environmental factors such as climate and vegetation;23 however, the duration of the cycle under controlled conditions (28±3⁰C and 80±5% RH) has an approximate duration of 49 to 81 days.24
Transmitted diseases
Ticks are the second most important vector worldwide, after mosquitoes, and are carriers of causative or infectious agents of diseases, called tick borne diseases (TBD).5 TBD incidence has increased through the spread of ticks linked to climatic changes. Rodríguez et al.,25 made a conglomerate of global reports of diseases related to tick bites with a geographical distribution that included countries such as the US, UK, Russia, France, Argentina, Brazil, Spain, and Israel, among others. NCEZID,26 in turn, reported on the increase of cases of Lyme disease that has occurred in the US from 2016 to 2017, from 26,203 to 29,513. In addition, it has been estimated that effects from ticks are seen in 80% of the cattle population worldwide.27
These diseases can be transmitted from ticks to vertebrates,4 the more important being the Jiangmen tick virus (JMTV), Lihan tick virus (LITV) and Wuhan tick virus (WTV-2), among other viruses,28-30 bacteria, such as rickettsia (Rickettsia spp), causative agents of spotted rocky mountain fever, and spotted Mediterranean fever,31,32 and Anaplasma marginale, which is the causative agent of bovine anaplasmosis and human granulocytic anaplasmosis.33,34 The bacteria transmitted by ticks include spirochetes (Borrelia spp), which cause Lyme disease, recurrent fever and rash disease, associated with the southern tick.35,36
Economic importance
The cattle tick has a great economic impact on the livestock industry through blood feeding and the transmission of pathogens. According to Meng & Sluder,23 a reliable number of economic losses worldwide caused by cattle ticks was indicated by Brown & Askenase,37 who reported economic losses estimated at US $8 billion caused by ticks in 1984; however, Betancur Hurtado & Giraldo-Ríos,38 observed US $18.7 million in annual losses caused by R. (B.) microplus worldwide. Several authors have presented varying values depending on the country (Table 1). Finally, Lew-Tabor & Rodríguez Valle,39 stated that the total estimated economic loss per animal per year (production plus control cost) can reach US $22-30 thousand per year.
|
Country |
Losses in $ USD |
Author/Year |
|
Brazil |
$3,240,000,000 |
Grisi et al.,40 |
|
USA |
$3,000,000,000 |
Graham & Hourrigan41 |
|
Mexico |
$573,610,000 |
Rodríguez-Vivas et al.,42 |
|
India |
$498,700,000 |
Senbill et al.,24 |
|
Australia |
$62,000,000 |
Manjunathachar et al.,27 |
|
Colombia |
$25,300,000 |
Puerta et al.,43 |
|
Puerto Rico |
$6,700,000 |
Senbill et al.,24 |
|
Zambia |
$5,000,000 |
Senbill et al.,24 |
Table 1 Report of economic losses caused by R. (B.) microplus, according to different sources.
Historically, tick infestations in cattle have been controlled using chemical acaricides with various active ingredients and application methods, such as immersion or sprinkling.23 This type of control began at the end of the 19th century with the use of arsenic and then advanced to formulations produced from active ingredients such as organochlorines, organophosphates, carbamates, amidines, pyrethroids, phenylpyrazoles, cyclic lactones, and growth regulators of insects and isoxazolines (Table 2).8,23,44-46
|
Active Ingredient/year of introduction23 |
Formulations |
Action Mechanism |
IRAC Grouping according to the action mechanism and point |
|
Arsenic 1895 |
Arsenic Trioxide, Potassium Arsenite, Dihydro-1, 3, 2, -dithiarsenol-2-mercaptoacetic acid |
Inhibit pyruvate dehydrogenase, competing with phosphate, decoupling oxidative phosphorylation, causing reduced energy-linked nicotinamide dinucleotide, mitochondrial respiration and the synthesis of adenosine triphosphate, leading to death.47 |
Pyruvate dehydrogenase (NADP +)/respiratory system inhibitors |
|
Organophosphates 1955 |
Ethion, Chlorpyrifos, Chlorphenvinphs And Coumaphos |
Act at the synapse of nerve junctions and inhibit the activity of acetylcholinesterase irreversibly, producing continuous nerve discharges that cause paralysis and death.48 |
Acetylcholinesterase/nervous system inhibitors |
|
Carbamates 1956 |
Carbaryl, Aldicarb, Carbofuran, Ethienocarb, Fenobucarb, Oxamyl, Propoxur |
||
|
Organochlorines 1939 |
Chlorinated Ethane Derivatives: DDT, |
Binding to the picrotoxin site in the gamma aminobutyric acid chloride (Cl -) ionophores complex (GABA), which inhibits the flow of Cl into the nerve and causes hyperexcitation and death49 |
GABA receptor (chlorine channel)/nervous system antagonists |
|
DDE (Dichloro-diphenyldichloro-ethane) and DDD (Dicofol, Methoxychloro) |
|||
|
Cyclodiene, Chlordane, Aldrine, Dieldrin, Hepatochlor, Endrin, Toxaphene. |
|||
|
Hexachlorocyclohexanes (HCH): Benzene Hexachloride (BHC) that includes γ-isomer, lindane |
|||
|
Pyrethroids 1978 |
Cypermethrin, Deltamethrin, Cyhalotrin and Flumethrin |
Block the movement of sodium ions along the axon of the nerve fiber. Stimulate repetitive nerve discharges that lead to paralysis and death.50 |
|
|
Phenylpyrazoles 1995 |
Fipronil, Pyriprole |
Binds to the allosteric sites of the GABAA and GluCl channels, acting as an antagonist (non-competitive inhibition), which prevents the opening of the Cl channels normally promoted by GABA49 |
|
|
Isoxazolines 2013 |
Afoxolaner, Fluralaner, Sarolaner, Lotilaner, CPD I |
Non-competitive GABA receptor antagonists that bind to Cl-channels in nerve and muscle cells blocking the transmission of neuronal channels, paralysis and death.49 |
|
|
Amidines 1975 |
Amitraz, Clordimeform, Clenpirin, Chloromethurgon |
Competing with octopamine for its receptor site, guanosine diphosphate is replaced by guanosine triphosphate, which induces the production of cyclic adenosine monophosphate that leads to inhibition of binding and, finally, to blood feeding, with the final death.47 |
Octopamine/nervous system receptor agonist |
|
Cyclic Lactones 1981 |
Avermectin: Doramectin, Selamectin, Abamectin, Ivermectin and Eprinomectin. |
Bind to GABA and glutamate-regulated chloride channels (GluCl), which opens chloride channels in the nerves, resulting in disruption of activity and loss of function in these cells that lead to paralysis and Death.51 |
Nicotinic acetylcholine receptor agonists/antagonists |
|
Milbemycins: Moxidectin, Milbemycin Oxima |
|||
|
Spinosyns: Spinosad |
|||
|
Growth Regulators 1994 |
Chitin synthesis inhibitors (Benzoylphenylureas), |
Structural resemblance to the molting hormone, 20-hydroxyecdsyone, thus interrupting the molting, metamorphosis and development of the female reproductive system. Surviving ticks are unable to produce a progeny.45 |
Chitin biosynthesis inhibitors-Acetyl CoA carboxylase inhibitors/hormone imbalance |
|
Chitin inhibitors (Triazine/Pyrimidine derivatives) |
|||
|
Juvenile Hormone Analogs |
|||
Table 2 Introduction, mode and point of action of active ingredients used as acaricides worldwide.
Resistance to chemical control
The intensive use of chemical acaricides has resulted in populations of ticks that exhibit resistance, understood as a characteristic or set of specific inherited traits resulting from the contact of said population with an acaricide, which results in significant increases of the percentage of the population that survives exposure to a certain concentration.42 In 2019, the Insecticide Resistance Action Committee (IRAC)47 defined resistance as an inheritable change in the sensitivity of a population of a pest that is reflected in repeated failures of a product to reach the expected levels of control when used in accordance with the label recommendations for that pest.
The evaluation of the effectiveness of these products has shown the time that has elapsed from introduction until the development of resistances. Meng & Sluder23 developed a timeline (Figure 1) that shows the years that have elapsed since introduction of an acaricide until the first report of resistance. The first evidence of a population of resistant ticks was presented by Mackerras,53 who, based on evidence received 10 years earlier from the Ayr district in Queensland (Australia), reported a decrease in the number of dead ticks after arsenic immersion baths, a situation that, according to the same author, was seen in South Africa and Argentina.
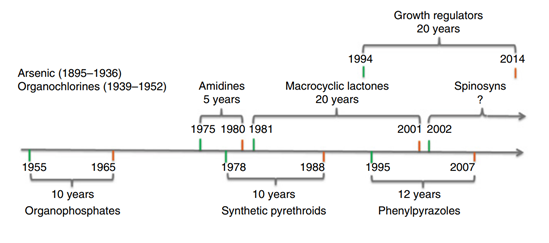
Figure 1 Chronological order of the introduction of acaricides used for tick control (green marker date) and the first resistance report according to the respective group (red marker date).23
The review by Adenubi et al.,12 in 2018, which has been modified by the authors, including reports from 2019 (Table 3), showed that there are 204 studies that show resistance to different active ingredients and formulations in R. (B.) microplus, with 134 of the studies from the Americas, where Mexico has the highest number of resistance reports, 42 in total, followed by Brazil (27) Australia (23), India (18) and Colombia (17). On the other hand, the active ingredient with the highest number of reports is cypermethrin.
|
Continent |
Country |
Reference |
Year |
Acaricide or active ingredient |
|
Africa |
Benin |
Adehan et al.,54 |
2016 |
Alpha-cypermethrin |
|
Africa |
Benin |
Adehan et al.,54 |
2016 |
deltamethrin |
|
Africa |
Benin |
Adehan et al.,54 |
2016 |
Amitraz |
|
Africa |
Egipto |
Aboelhadid et al.,50 |
2018 |
deltamethrin |
|
Africa |
South Africa |
Ntondini et al.,55 |
2008 |
Amitraz |
|
Africa |
South Africa |
Ntondini et al.,55 |
2008 |
Cypermethrin |
|
Africa |
South Africa |
Ntondini et al.,55 |
2008 |
chlorfenvinphos |
|
Africa |
South Africa |
Baron et al.,56 |
2015 |
Amitraz |
|
Africa |
South Africa |
Lovis et al.,57 |
2013 |
Pyriprol |
|
Africa |
South Africa |
Lovis et al.,57 |
2013 |
Cypermethrin |
|
Africa |
South Africa |
Lovis et al.,57 |
2013 |
fenvalerate |
|
Africa |
Tanzania |
Kagaruki58 |
1991 |
Dieldrin |
|
Africa |
Tanzania |
Kagaruki58 |
1991 |
lindane |
|
Africa |
Zambia |
Muyobela et al.,59 |
2015 |
Amitraz |
|
Africa |
Zambia |
Muyobela et al.,59 |
2015 |
Cypermethrin |
|
S America |
Argentina |
Mangold et al.,59 |
2004 |
Flumethrin |
|
S America |
Argentina |
Cutullé et al.,60 |
2013 |
Amitraz |
|
S America |
Argentina |
Cutullé et al.,60 |
2013 |
Cypermethrin |
|
S America |
Argentina |
Cutullé et al.,60 |
2013 |
Flumethrin |
|
S America |
Argentina |
Lovis et al.,61 |
2013 |
Amitraz |
|
S America |
Argentina |
Lovis et al.,61 |
2013 |
Cypermethrin |
|
S America |
Argentina |
Lovis et al.,61 |
2013 |
Flumethrin |
|
S America |
Argentina |
Cutullé et al.,60 |
2013 |
Amitraz |
|
S America |
Argentina |
Cutullé et al.,60 |
2013 |
deltamethrin |
|
S America |
Bolivia |
Villarroel-Alvarez et al.,62 |
2006 |
Flumethrin |
|
S America |
Bolivia |
Villarroel-Alvarez et al.,62 |
2006 |
deltamethrin |
|
S America |
Bolivia |
Villarroel-Alvarez et al.,62 |
2006 |
Cypermethrin |
|
S America |
Brazil |
Martins & Furlong63 |
2001 |
Doramectin |
|
S America |
Brazil |
Martins & Furlong63 |
2001 |
moxidectina |
|
S America |
Brazil |
Li et al.,64 |
2004 |
Amitraz |
|
S America |
Brazil |
Klafke et al.,6 |
2006 |
Ivermectin |
|
S America |
Brazil |
Mendes et al.,65 |
2007 |
Cypermethrin |
|
S America |
Brazil |
Mendes et al.,65 |
2007 |
deltamethrin |
|
S America |
Brazil |
Mendes et al.,65 |
2007 |
Chlorpyriphos |
|
S America |
Brazil |
Castro-Janer et al.,49 |
2010 |
Fipronil |
|
S America |
Brazil |
Klafke et al.,6 |
2010 |
Ivermectin |
|
S America |
Brazil |
Klafke et al.,6 |
2011 |
Ivermectin |
|
S America |
Brazil |
Andreotti et al.,66 |
2011 |
Alpha-cypermethrin |
|
S America |
Brazil |
Andreotti et al.,66 |
2011 |
Cypermethrin |
|
S America |
Brazil |
Andreotti et al.,66 |
2011 |
Amitraz |
|
S America |
Brazil |
Mendes et al.,67 |
2011 |
Deltamethrin |
|
S America |
Brazil |
Mendes et al.,67 |
2011 |
Chlorpyriphos |
|
S America |
Brazil |
Mendes et al.,67 |
2011 |
Cypermethrin |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
Chlorpyriphos |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
Amitraz |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
Cypermethrin |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
fipronil |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
Ivermectin |
|
S America |
Brazil |
Reck et al.,68 |
2014 |
Fluazuron |
|
S America |
Brazil |
Klafke et al.,6 |
2016 |
Amitraz |
|
S America |
Brazil |
Klafke et al.,6 |
2016 |
Chlorpyriphos |
|
S America |
Brazil |
Klafke et al.,6 |
2016 |
Cypermethrin |
|
S America |
Brazil |
Klafke et al.,6 |
2016 |
Fipronil |
|
S America |
Brazil |
Klafke et al.,6 |
2016 |
ivermectin |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
Cypermethrin |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
deltamethrin |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
coumaphos |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
chlorfenvinphos |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
diazinon |
|
S America |
Colombia |
Benavides et al.,69 |
2000 |
Amitraz |
|
S America |
Colombia |
Diaz & Vallejo70 |
2013 |
Cypermethrin |
|
S America |
Colombia |
Lopez-Arias et al.,71 |
2014 |
Cypermethrin |
|
S America |
Colombia |
Lopez-Arias et al.,71 |
2014 |
Amitraz |
|
S America |
Colombia |
Araque et al.,72 |
2014 |
Amitraz |
|
S America |
Colombia |
Araque et al.,72 |
2014 |
ethion |
|
S America |
Colombia |
Puerta et al.,43 |
2015 |
Cypermethrin |
|
S America |
Colombia |
Puerta et al.,43 |
2015 |
Amitraz |
|
S America |
Colombia |
Villar et al.,73 |
2016a |
Ivermectin |
|
S America |
Colombia |
Villar et al.,74 |
2016b |
Deltamethrin |
|
S America |
Colombia |
Villar et al.,74 |
2016b |
Amitraz |
|
S America |
Colombia |
Villar et al.,74 |
2016b |
Chlorpyriphos |
|
N America |
Costa Rica |
Hagen et al.,75 |
1999 |
Flumethrin |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
Chlorpyriphos |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
coumaphos |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
Flumethrin |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
deltamethrin |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
ivermectin |
|
N America |
Costa Rica |
Alvarez & Hernandez76 |
2010 |
Amitraz |
|
N America |
Cuba |
Valdez et al.,77 |
1999 |
Chlorfenvinphos Cyamizol |
|
N America |
El Salvador |
Hagen et al.,75 |
1999 |
Flumethrin |
|
N America |
Guatemala |
Hagen et al.,75 |
1999 |
Deltamethrin |
|
N America |
Guatemala |
Hagen et al.,75 |
1999 |
Flumethrin |
|
N America |
Guatemala |
Hagen et al.,75 |
1999 |
cyfluthrin |
|
N America |
Jamaica |
Rawlins & Mansingh78 |
1978 |
Carbaryl |
|
N America |
Jamaica |
Rawlins & Mansingh78 |
1978 |
Lindane |
|
N America |
Jamaica |
Rawlins & Mansingh78 |
1978 |
chlorfenvinphos |
|
N America |
México |
Ortiz et al.,79 |
1995 |
Dieldrin |
|
N America |
México |
Ortiz et al.,79 |
1995 |
Cypermethrin |
|
N America |
México |
Ortiz et al.,79 |
1995 |
deltamethrin |
|
N America |
México |
Ortiz et al.,79 |
1995 |
Lindane |
|
N America |
México |
Ortiz et al.,79 |
1995 |
coumaphos |
|
N America |
México |
Ortiz et al.,79 |
1995 |
diazinon |
|
N America |
México |
Ortiz et al.,79 |
1995 |
dioxathion |
|
N America |
México |
Ortiz et al.,79 |
1995 |
dimethoate |
|
N America |
México |
Ortiz et al.,79 |
1995 |
ethion |
|
N America |
México |
Fragoso et al.,80 |
1995 |
Amitraz |
|
N America |
México |
Soberanes et al.,81 |
2002 |
Amitraz |
|
N America |
México |
Li et al.,64 |
2004 |
Carbaryl |
|
N America |
México |
Rodriguez-Vivas et al.,82 |
2006b |
Amitraz |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
Diazinon |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
coumaphos |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
chlorfenvinphos |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
Flumethrin |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
deltamethrin |
|
N America |
México |
Rodríguez-Vivas et al.,83 |
2007 |
Cypermethrin |
|
N America |
México |
Rosado-Aguilar et al.,84 |
2008 |
Amitraz |
|
N America |
México |
Fernández-Salas et al.,85 |
2012c |
Cypermethrin |
|
N America |
México |
Fernández-Salas et al.,86 |
2012b |
Diazinon |
|
N America |
México |
Fernández-Salas et al.,86 |
2012b |
Flumethrin |
|
N America |
México |
Fernández-Salas et al.,86 |
2012b |
deltamethrin |
|
N America |
México |
Fernández-Salas et al.,86 |
2012b |
Cypermethrin |
|
N America |
México |
Perez-Cogollo et al.,87 |
2010a |
Ivermectin |
|
N America |
México |
Rodríguez-Vivas et al.,88 |
2011 |
Cypermethrin |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
Amitraz |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
Flumethrin |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
deltamethrin |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
Cypermethrin |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
Chlorpyriphos |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
coumaphos |
|
N America |
México |
Olivares-Pérez et al.,89 |
2011 |
diazinon |
|
N America |
México |
Miller et al.,90 |
2013 |
Fipronil |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
Ivermectin |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
Amitraz |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
Chlorpyriphos |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
coumaphos |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
Cypermethrin |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
permethrin |
|
N America |
México |
Rodríguez-Vivas et al.,91 |
2013 |
fipronil |
|
N America |
Panama |
Hagen et al.,75 |
1999 |
Flumethrin |
|
N America |
Panama |
Torrijos et al.,92 |
2015 |
Cypermethrin |
|
N America |
Dominican Republic |
Hagen et al.,75 |
1999 |
Deltamethrin |
|
N America |
Dominican Republic |
Hagen et al.,75 |
1999 |
Flumethrin |
|
N America |
Dominican Republic |
Hagen et al.,75 |
1999 |
cyfluthrin |
|
S America |
Uruguay |
Castro-Janer et al.,93 |
2009 |
Fipronil |
|
S America |
Uruguay |
Castro-Janer et al.,94 |
2011 |
Ivermectin |
|
S America |
Uruguay |
Cuore & Solari95 |
2014 |
Ethion |
|
S America |
Uruguay |
Cuore & Solari95 |
2014 |
Cypermethrin |
|
S America |
Uruguay |
Cuore & Solari95 |
2014 |
Amitraz |
|
S America |
Uruguay |
Cuore & Solari95 |
2014 |
fipronil |
|
S America |
Uruguay |
Cuore & Solari95 |
2014 |
ivermectin |
|
S America |
Uruguay |
Castro-Janer et al.,96 |
2015 |
Fipronil |
|
S America |
Uruguay |
Castro-Janer et al.,96 |
2015 |
Lindane |
|
N America |
USA |
Miller et al.,97 |
2007b |
Permethrin |
|
N America |
USA |
Busch et al.,98 |
2014 |
Coumaphos |
|
N America |
USA |
Busch et al.,98 |
2014 |
permethrin |
|
N America |
USA |
Busch et al.,98 |
2014 |
Amitraz |
|
N America |
USA |
Busch et al.,98 |
2014 |
ivermectin |
|
N America |
USA |
Busch et al.,98 |
2014 |
fipronil |
|
N America |
USA |
Klafke et al.,6 |
2017 |
permethrin |
|
N America |
USA |
Klafke et al.,6 |
2017 |
Cypermethrin |
|
N America |
USA |
Klafke et al.,6 |
2017 |
deltamethrin |
|
N America |
USA |
Klafke et al.,6 |
2017 |
Flumethrin |
|
S America |
Venezuela |
Coronado99 |
1999 |
Amitraz |
|
Asia |
India |
Chaudhuri & Naithani100 |
1964 |
BHC |
|
Asia |
India |
Kumar et al.,101 |
2011 |
Diazinon |
|
Asia |
India |
ALT Sharma et al.,102 |
2012 |
Deltamethrin |
|
Asia |
India |
ALT Sharma et al.,102 |
2012 |
Cypermethrin |
|
Asia |
India |
Shyma et al.,103 |
2013 |
Deltamethrin |
|
Asia |
India |
Shyma et al.,103 |
2013 |
Cypermethrin |
|
Asia |
India |
Shyma et al.,103 |
2013 |
diazinon |
|
Asia |
India |
Singh et al.,104 |
2014 |
Cypermethrin |
|
Asia |
India |
Jyoti Singh et al.,105 |
2014 |
Malathion |
|
Asia |
India |
Singh et al.,106 |
2015 |
Amitraz |
|
Asia |
India |
Ghosh et al.,107 |
2015 |
Deltamethrin |
|
Asia |
India |
Ghosh et al.,107 |
2015 |
diazinon |
|
Asia |
India |
Shyma et al.,108 |
2015 |
Deltamethrin |
|
Asia |
India |
Shyma et al.,108 |
2015 |
fipronil |
|
Asia |
India |
Shyma et al.,108 |
2015 |
Flumethrin |
|
Asia |
India |
Gaur et al.,109 |
2016 |
Deltamethrin |
|
Asia |
India |
Gaur et al.,109 |
2016 |
diazinon |
|
Asia |
India |
Khangembam et al.51 |
2018 |
ivermectin |
|
Asia |
Iran |
Ziapour et al.,1 |
2016 |
lambda-cyhalothrin |
|
Asia |
Iran |
Ziapour et al.,1 |
2016 |
Cypermethrin |
|
Australia |
Australia |
Mackerras53 |
1936 |
arsenic tetroxide |
|
Australia |
Australia |
Stone & Webber110 |
1960 |
BHC |
|
Australia |
Australia |
Stone & Webber110 |
1960 |
DDT |
|
Australia |
Australia |
Stone & Webber110 |
1960 |
dieldrin |
|
Australia |
Australia |
Stone & Meyers110 |
1957 |
Dieldrin |
|
Australia |
Australia |
Shaw111 |
1966 |
Carbophenothion |
|
Australia |
Australia |
Shaw111 |
1966 |
dioxathion |
|
Australia |
Australia |
Shaw111 |
1966 |
diazinon |
|
Australia |
Australia |
Shaw111 |
1966 |
parathion |
|
Australia |
Australia |
Shaw111 |
1966 |
carbaryl |
|
Australia |
Australia |
Nolan et al.,112 |
1989 |
Cypermethrin |
|
Australia |
Australia |
Nolan et al.,112 |
1989 |
cyhalothrin |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
Dimethoate |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
dioxathion |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
coumaphos |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
cyanophos |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
Chlorpyriphos |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
dieldrin |
|
Australia |
Australia |
Roulston et al.,113 |
1981 |
DDT |
|
Australia |
Australia |
Jonsson & Hope114 |
2007 |
Amitraz |
|
Australia |
Australia |
Lovis et al.,57 |
2013 |
Flumethrin |
|
Australia |
Australia |
Lovis et al.,57 |
2013 |
Cypermethrin |
|
Australia |
Australia |
Lovis et al.,57 |
2013 |
pyriprol |
|
Australia |
New Caledonia |
Brun et al.,115 |
1983 |
Ethion |
|
Australia |
New Caledonia |
Beugnet & Chardonnet116 |
1995 |
Fenvalerate |
|
Australia |
New Caledonia |
Beugnet & Chardonnet116 |
1995 |
deltamethrin |
|
Australia |
New Caledonia |
Beugnet & Chardonnet116 |
1995 |
Flumethrin |
|
Australia |
New Caledonia |
Bianchi et al.,117 |
2003 |
Deltamethrin |
|
Australia |
New Caledonia |
Bianchi et al.,117 |
2003 |
ethion |
|
Australia |
New Caledonia |
Ducornez et al.,118 |
2005 |
Amitraz |
|
Australia |
New Caledonia |
Petermann et al.,119 |
2016 |
deltamethrin |
|
Australia |
New Caledonia |
Petermann et al.,119 |
2016 |
Amitraz |
Table 3 Historical reports of resistance generated by R. (B.) microplus worldwide
The latest reports on resistance in R. (B.) microplus include resistance to deltamethrin, where the use of the recommended dose (200 ppm) caused 33.33% mortality in ticks, and the application of double the recommended dose caused 56% mortality, while for the use of ivermectin, there are high resistance factors in treatments performed both in the laboratory and in the field.50,51 These and other reports on ticks with resistance to different chemical acaricide formulations elucidate the difficulty that comes with the development of new molecules capable of exercising efficient control.120
Factors that lead to the development of resistance
The FAO, in 2013,121 established that the development of resistance is not only linked to operational factors but is also largely due to genetic and biological factors of the pest. In arthropods, development is partly dependent on factors related to the use of acaricides and the life cycle of the organism.122 Therefore, internal and external factors influence the development of resistance in R. (B.) microplus.
Biological factors refer to the duration of the life cycle, population densities, reproductive capacity, type of reproduction and host range of the pest.121 R. (B.) microplus, which is a single host tick, has a shorter life cycle than the ticks of several hosts and produces a high amount of eggs, which means that this species produces a greater number of offspring annually. Therefore, a large variety of acaricides is needed to effectively control infestations,122 resulting in a high potential for resistance development.
A clear explanation of the operational activities that lead to the development of resistance in cattle ticks was given by Vudriko et al.123, who reported that Ugandan producers employ activities that are not effective in the long term, with an increase of 2 to 4 times the concentrations in applications and an increase in the frequency of the use of acaricides, along with mixing two or more acaricides and not correctly rotating the products that are used, activities that are contrary to the integrated management approach for pests as presented by the Insecticide Resistance Action Committee47 and the FAO for several years.
Rodríguez-Vivas et al.,52 studied the genetic behavior of resistance in R. (B.) microplus according to phenotype and genotype, following the distinction made by Guerrero et al.,122 who established that the resistant phenotype is given by the susceptibility or resistance of a group of individuals to the effects of an application of a certain acaricide, while the resistance genotype refers to the genetic composition of the tick, which leads to the expression of the resistance phenotype.
These genetic factors occur because of the modification of a specific gene or group of genes linked to responses that will prevent the expected acaricidal effect from occurring in the pest.121 In order to understand the way in which genetics acts in the development of different types of resistance, studies have been carried out that associate genetic alterations with the resistance mechanism.
Resistance mechanisms
Genetic changes promote the development of resistance in tick populations. These changes generate resistance mechanisms in individuals, described as follows: modifications at the target site, increased metabolism, acaricide sequestration, or reduced ability of acaricide to penetrate through the outer protective layers of the tick body (Table 4).122
In the identification of resistance mechanisms in R. (B) microplus, there have been advances that show the way in which the enzymatic, metabolic, genetic and proteomic activities are involved (Table 4). There are also reports that have demonstrated the presence of combinations of different resistance mechanisms.96,125,126,6 This type of resistance is known as "cross resistance," which occurs when a single defense mechanism against an insecticide also confers resistance to other insecticides, even if the insect has not been previously exposed to the other products.130
|
Resistance mechanism |
Definition |
Resistance studies on R. B. microplus |
|
|
Metabolic detoxification (enzymatic) |
Development of high levels of a particular enzyme or altered forms of it with higher catalytic rates, which eliminate naturally occurring toxins in hosts, these enzymes include esterases, cytochrome P450 monooxygenases, and glutathione S-transferases.124 |
Increase in the activity of the enzymes β-naphthol, β-esterase and cytochrome P450, in pyrethroid, organophosphorus and phenylpyrazole resistant ticks.125 |
|
|
Reduced sensitivity at the site of action |
Change of pesticide fixation site, eliminating or significantly reducing effectiveness121 |
KDR (shock resistance): sodium channel interference in nerve cells. Commonly developed in resistance to DDT and pyrethroids.47 |
Mutations in the s4-5 gene of domain II of the sodium channel, given by conversion of glycine to valine at position 72, generate resistance to DDT and malathion126 |
|
MACE (modified acetylcholinesterase): modifies the structure of acetylcholinesterase so that it is no longer affected by the insecticide.121 |
Development of resistance to organophosphorus by modified acetylcholinesterase as a result of fluctuations in the increase in transcription of the achE2 and pAChE2 genes48 |
||
|
RDL (resistance to dieldrin) is a mutation point that reduces the binding of dieldrin to the GABA receptor.49 |
Alanine substitutions (A286S / L) in populations resistant to fipronil49 |
||
|
Sequestration |
Metabolic enzymes increase considerably (up to 15% of the total body of the protein) and fixed to the insecticide, but the insecticide is not metabolized, that is, it is not sequestered.122 |
- |
|
|
Behavioral Resistance |
The modification of the behavior helps to avoid the lethal effect of the pesticides since feeding simply stops if the individual becomes close to some insecticides or can leave the area that has been treated.126 |
The study of behavioral resistance in R. (B). microplus has not been addressed, however Soares & Borges,127 studied the behavior of Amblyomma Cajennense, identifying sensory odor neurons that presented positive responses (movement towards) to 2,6-dichlorophenol (2,6-DCP) |
|
|
Reduced penetration |
This mechanism retards the penetration of the pesticide through the cuticle of resistant insects, producing low levels of resistance, which by delaying the penetration of the toxic through the cuticle greatly increases the impact of other resistance mechanisms.47 |
Kluck et al.,128 verified the presence of a candidate hemolymphatic protein, called Rhipicephalus microplus lipid carrier protein (RmLCP), this lipoprotein binds and transports free cholesterol and is presumed to be involved in lipid modification that promotes reduced penetration resistance |
|
Table 4 Types of resistance generated by R. (B.) microplus
This species has been a favorite organism for evaluating different types of extracts worldwide, given its distribution and importance in animal and human health. Therefore, these evaluations are based on the effect that these extracts produce in adult, nymph and larval mortality, along with the effect on oviposition and hatching.12
There is a global trend to reduce the use of chemical insecticides and acaricides, mainly caused by the development of resistance and the presence of traces of chemical residues in food that damage human and animal health, The loss of biodiversity and ecosystem degradation, and costs are other drawbacks of the use of acaricides.8
Therefore, in order to control tick infestations, alternatives for their control have been developed, based on the use of plants that are recognized for their antiparasitic characteristics131 and that generally have a lower value and are safer and more friendly to the environment, promoting interest in the use and study of extracts obtained from these plants.132
Worldwide there is a wide variety of plant extracts of various species, prepared through different methodologies that use leaves, roots, stems, flowers, fruits and seeds, obtaining extracts based on water,9,131 oil133 or different types of alcohols.11,132,134 Essential oils have been obtained and used135,136 in spray dried powder6 for the control of this important ectoparasite.
According to a review by Adenubi et al.,8 in the use of plant extracts for the control of different species of ticks worldwide from 1914 to 2014, 30 species of plants were used. Two years later, Adenubi et al.,12 found that the number of species used for the elaboration of extracts with a tick effect increased from 30 to 55. These extracts are distributed as follows: according to the families to which they belong: Lamiaceae 20%, Asteraceae 13%, Rutaceae and Fabaceae 9% and Solanaceae 7%. This study clarified that, of the studies conducted on bio-extracts for the control of ticks, only 17% focused on how these extracts act or their mode of action, which should be addressed since this information theoretically and scientifically reinforces the use of plant extracts in the control of pests and diseases.
The presence of secondary metabolites that are related to the control of ticks has been evidenced from plant extracts, including flavonoids, terpenes, spilanthol and coumarins.13,137-139 The latter are the most important within this document since they are one of the largest classes of natural compounds and are present in many plants as secondary metabolites in roots, stems, leaves, branches and seeds.140
Development and employment of coumarins
Coumarins have been thoroughly studied because of their potential for fighting diseases and pests, both in plants and animals, and their anti-inflammatory, antioxidant, antimicrobial, antiviral, anticoagulant and anticancer activities.141,142 This family of secondary plant-derived metabolites was first isolated in 1820 by Voguel from a Fabaceae called Coumarouna odorata, now known as Dipteryx odorata, commonly known as cumarú.142 Its main use was the cosmetic industry.143
It took 40 years for Perkin in 1868 to design a reaction using salicylaldehyde and acetic anhydride to synthesize coumarin, a methodology that is still used today.142 In 1947, Seshadri & Murti144 compared coumarin derivatives to natural flavones to reveal the level of toxicity of these compounds because they had previously been associated with hemorrhagic diseases in cattle, resulting in 3-phenyl and 4-phenylumbelliferone and its methyl esters, which are highly toxic in fish. By 1948, the efforts of Karl Link145 and his collaborators took the use of coumarins a step further when warfarin (3-phenylacetyl ethyl, 4-hydroxycoumarin) was isolated from coumarin compounds, which was used as rodenticide, the most potent form of synthetic coumarins at the time.146 Warfarin, in addition to being used for rodenticide, was also included in the prevention of thromboembolic diseases, demonstrating greater efficiency than dicumarol and heparins.147
On March 5, 1954, the use of coumarins in food148 was regulated because liver diseases related to high doses of coumarins were reported, where prolonged doses of 2500 ppm caused pathologies in the livers of mice.149,150 In the 1960s, significant advances were reported for the use of different types of coumarins as growth regulators in plants, such as antispasmodic and analgesic agents, breathing stimulants, vasodilators, antibacterial, anthelmintic, and antifungal agents, and insecticides;151 many others are still studied today. The study of biosynthetic routes for coumarins began with Kosuge & Conn in 1959,152 who demonstrated that the shikimic acid route is the pathway for the metabolization of o-coumaric acid, which is a precursor of Coumarins and is associated with the synthesis of the aromatic ring of the molecule. Another study was carried out by Brown during the 60s and 70s that focused on the study of the biosynthetic routes of coumarins, conducting experiments that identified precursors involved in the formation of coumarins, such as L-phenylalanine and p-coumaric acid, and pointing out reactions such as methylation, lactonization, and hydroxylation, among others.153,154
Parallel to these investigations and as the result of the development of resistance to the use of these compounds in anticoagulant treatments and in the use as rodenticide, from 1975 to 1978, the second generation of coumarins was developed,155 which were based on the replacement of stereochemically similar side chains in the 4 hydroxycoumarin, known as difenacoum and brodifacoum.156 In the late 1980s, research on coumarins focused on new ways of synthesizing the molecule and its use in medicine, with contributions from Harvey et al.,157 who, in 1988, developed a new type of synthesis based on Ortho-directed methylation, obtaining a series of coumarins with substituents in positions 6 and 7, which were used in bioassays to reveal their antitumor activity, resulting in polycyclic coumarins with a chemo preventive cancer activity.
By 2002, Lake et al.,158 demonstrated with live studies in both mice and Drosophila melanogaster that coumarin compounds are not genotoxic agents. However, in vitro tests on human liver cultures with high doses of coumarins resulted in genotoxicity. Kostova159 reported a considerable number of tests carried out in vivo and in vitro on cytotoxicity related to the use of coumarins, which may present a greater or lesser degree of cytotoxicity according to the type of substituent that is used.160
Research on the possible uses and characteristics of coumarins are still valid. In fact, there are a large number of reports aimed at alternative uses of coumarins; more than 1300 coumarins have been isolated from plants, bacteria and fungi,14-16 with antioxidant,161 anti-HIV,162 anticancer,163 antiviral,164 antituberculosis,165 insecticide and fungicide properties,166 among others.
Obtaining coumarins
Natural coumarins
Coumarins occur naturally in plants. These secondary metabolites are associated with defense functions against fungi and insects, Coumarin compounds are a class of lactones structurally constructed by a benzene ring fused with an α-pyrone ring.167
Coumarins are naturally synthesized phenolic compounds, through the shikimic acid pathway. This metabolic pathway occurs from the reaction of l-phenylalanine, which is catalyzed by the enzyme phenylalanine ammonium lyase (PAL), resulting in cinnamic acid. Once this acid has been formed, a series of reactions that includes hydroxylation, methylation and dehydration occurs, the most important being the reactions that take place in the ortho and para positions, which in turn involve processes in which enzymes intervene, such as cinnamate 4 hydroxylase, in the putative P450 and P450 metabolic pathways, along with the independent routes of these enzymes and others, in which the lactonization process occurs, resulting in various types of coumarins.168,169
Voguel, in 1820, was the first to report the successful extraction of coumarins from Dipteryx odorata;142,151 however, it was not until years later that the methodologies for obtaining them were known,170 using different species of plants146 and different plant parts. These methodologies are based on obtaining plant extracts from the maceration of plant material and the addition of solvents of increasing polarity, such as petroleum ether and bicarbonate or sodium carbonate, which then allow direct crystallization from the concentrated extract, either during Soxhlet extraction or at rest at a higher concentration and cooling of the extract.171
Currently, the isolation of coumarins is carried out with techniques that combine different types of maceration, either using ultrasound, liquid nitrogen, infusions, or solvents of different polarities, such as hexane, chloroform, ethyl acetate and methanol, resulting in concentrates with vacuum distillation using Soxhlet, supercritical CO2 extraction,172 pressurized hot water extraction,173 microwave-assisted extraction,174 or dispersion in the solid phase of the effervescence-assisted matrix (EA-MSPD).175
Synthesis of coumarins
Research developed with the objective of synthesizing new coumarins has been widely addressed. The first methodology for obtaining coumarins was proposed by Perkin in 1868.142 Perkin used different temperatures, an alkaline salt as a catalyst, and acetic acid, which resulted in a type of aldol condensation from an interaction between a carbanion and a carboxyl group.176,177 This was followed by methodologies such as condensation by Knoevenagel, which used the reaction between an aldehyde or its derivative and an ester, in the presence of the amine as a catalyst. This reaction synthesizes coumarins through cyclisation of the lactone group without the presence of solvents in processes carried out with microwaves178 or the Reformatsky reaction, which generates the condensation of aldehydes (or ketones) with α-halo esters in the presence of metallic zinc, forming β-hydroxy esters that are dehydrated in subsequent steps to produce an unsaturated ester.179,180
On the other hand, the Wittig reaction synthesizes an alkene from the reaction of an aldehyde or ketone with the ilium generated from a phosphonium salt.181 Claisen also developed a decarboxylative condensation reaction, where, starting from an ester and a strong base used as a catalyst, a single carbon-carbon bond is produced.182 Pechmann used the esterification/transesterification of phenols with β-ketoesters with acids such as Bronsted or Lewis.183,184 These methodologies laid the foundation for the development of processes that obtain coumarin compounds.171 These new methodologies are based on the use of different reactions and catalytic processes in which the precursors and materials used to obtain coumarins vary, involving ecological approaches, new technologies, such as microwaves184 and ultrasound,185 new catalysts,186,187 ecological solvents,188 reactions without Solvents189 and molecular coupling,190,191 among others, resulting in coumarins with greater yields and better activity, demonstrating the importance of coumarin compounds in different industries and providing coumarin derivatives in recent years that are much more easily obtained, economical and environmentally responsible.
Use of coumarins for tick control
Many of the extracts evaluated for the control of ticks use coumarns; hence, they are associated with the effect on ticks. The cases in which mortality and/or repellency were greater than 60% were compiled, given that this effect resulted from the direct use of coumarins or by the presence of coumarins in bio-extracts.
Tunón et al.,192 evaluated an extract of Artemisia abrotanum obtained from toluene and the essential oil of Dianthus caryophyllum, which, based on identification with thin layer chromatography, contains coumarins. This extract produced 93% mortality rates in Ixodes ricinus nymphs after 4 hours from the start of the test.
Mortality values obtained from the use of Ocotea elegans essential oil in R. (B.) microplus larvae and adults were 70% mortality with a concentration of 100mg/ml in the larvae and, in the adults, were greater than 60% with a concentration of 6.2 mg/ml, along with 97% mortality at 25 mg/ml. Although no coumarins were detected in the essential oil of Ocotea elegans, Figueiredo et al.,138 it has been previously reported as containing them.193,194
It has been shown that the use of coumarins for the control of Rhipicephalus appendiculatus larvae has been successful, with up to 90% mortality when two types of coumarin compounds isolated from Acokanthera schimperi were mixed.17
On the other hand, Rosado-Aguilar et al.,195 stated that studies on the effects of essential oils and extracts of plants with coumarins showed an efficacy of 5 to 100%, with the genus Rhipicephalus being the most studied. In addition, tests on the inhibition of egg hatching showed efficiencies of 60-100%, and tests on larvae and adults produced mortality of 5 to 100% and 60 to 100%.132 The results obtained by Dantas et al.,132 and reported by Rosado-Aguilar et al.,195 confirmed that coumarins are a promising alternative for the control of ticks that are susceptible and resistant to conventional acaricides.
Mode of action of coumarins in ticks
Whatever the mechanism of action of coumarins, the strength of their binding to the target is increased by additional interactions involving the substituents present in the coumarin scaffold. The type of substituents and the substitution pattern determine, along with the general binding energy and potency, the selective interactions of coumarin derivatives with specific objectives.170
The use of plants with high tannin contents and the presence of cumaronochromones196 causes darkening of the cuticle, lack of movement in the Malpighi tubes and hemorrhagic skin lesions in R. (B.) microplus adults. A possible mode of action in coumarins was evidenced in a study by Enan,197 who evaluated the behavior of Periplaneta americana after being treated with three essential oils with cinnamic alcohol, which has been associated with coumarins by Ntalli et al.,198 resulting in some signs of toxicity, such as hyperactivity, followed by hyperextension of the legs and abdomen, then rapid immobilization and finally death, symptoms that were compared with treatments with induced octopamine, finding similar signs of toxicity.
Because of the capacity not only of this species but of other types of ticks to expand distribution worldwide, it is necessary to develop and subsequently implement strategies with high viability and easy acceptance by producers since the use of acaricides and control techniques based on chemical compounds have lost their level of effectiveness, causing an increase in costs in relation to the effectiveness in the control of this species.
The use of plant extracts obtained from different plant species through different techniques has proven promising for the control of R. (B.) microplus, demonstrating even better behavior than the active ingredients used today for the control of this pest; however, more research is needed for the point and mode of action that these extracts have on the individual, either at the biochemical, molecular, proteomic or physiological levels.
Although coumarins are present in several plant extracts obtained from different species, there are very few studied on their acaricidal activity, a topic of great interest for future research, which can take into account the methodologies and raw materials used for obtaining them and their mode of action in R. (B.) microplus since the effect they have depends on, among other things, their chemical structure.
The authors are thankful for the support of the Grupo de Investigación en Bioquímica y Nutrición Animal – GIBNA of the Universidad Pedagógica y Tecnológica de Colombia.
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.

©2020 Rodriguez-Molano, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.