eISSN: 2379-6367


Review Article Volume 6 Issue 3
1Department of Analytical Chemistry, Escuela Politecnica Superior, University of Seville, Spain
2Department of Analytical Chemistry, Faculty of Pharmacy, University of Seville, Spain
Correspondence: Julia Martin, Department of Analytical Chemistry, Escuela Politecnica Superior, University of Seville. C/Virgen de Africa, 7, E-41011 Seville, Spain, Tel 34-9-5455-6250
Received: March 26, 2018 | Published: May 30, 2018
Citation: Martin J, Alés MR, Asuero AG, et al. An overview on ligands of therapeutically interest. Pharm Pharmacol Int J. 2018;6(3):198-214. DOI: 10.15406/ppij.2018.06.00177
The principles governing metal-ligand complex stability and specificity depend on the properties of both the metal and the chelating agent. The exploration of coordination chemistry offers the real prospects of providing new understanding of intractable diseases and of devising novel therapeutics and diagnosis agents. Refinement in the approach to chelator design has come with a more subtle understanding of binding kinetics, catalytic mechanisms and donor interactions. Ligands that effectively bind metal ions and also include specific features to enhance targeting, reporting, and overall efficacy are driving innovation in areas of disease, diagnosis and therapy. In this contribution the topics of bioinorganic medicinal chemistry, chelating agents in the treatment of metallic ion overload in the body, and expanding the notion of chelating agent in medicine are successively dealt with, paying then attention to platinum, gold, iron, copper and aluminium ion metal complexes having medicinal interest. A tabular summary containing selected applications of ligands and complexes of therapeutic interest is also shown to including the most relevant and current bibliography. A number of papers concerning miscellaneous topics based on selected key words are also tabulated. Metal chelation principles offer wide new opportunities in the drug design field in addition to the classical answer of metal sequestration or elimination.
Keywords: ligands, complexes, therapeutic applications, new drugs
The principles governing metal-ligand complex stability and specificity depend on the properties of both the metal and the chelating agent.1‒4 Research in the field of inorganic medicinal chemistry has increased in the last two decades. In order to modify and control the features of metal ions in biological systems, a number of chelating ligand agents have been exploited.3,5 Metal ions show a vital function in the advance and pathology of a variety of ilness situations6‒8 and, in some cases, are implicated in redox chemistry leading to oxidative stress. Many disorders implie a high level of metal ions in given tissues or body cell compartments. Solution to those generated problems are challenging requiring the therapeutic answers among posible mediations the use of new chelator agents.9‒11
The wide success of platinum drugs has been promoting the development of both alternative platinum- and other non-platinum based compounds.12 The pharmaceutical industry has yet to appreciate the impact coordination chemistry can have on the design of new medicines. This may change in the future as skilled multidisciplinary practitioners develop their research using strategic approach to complex design. Refinement in the approach to chelator design has come with a more subtle understanding of biding kinetics, catalytic mechanisms and donor interactions.4,8,13 Rational ligand design offer to the medicinal chemistry, control over kinetic properties, such as the rate of ligand exchange. Our discussion is only a partial sample of the state of the art. Platinum, gold, iron, copper and aluminium complexes are mainly considered in this context. Summaries of papers related with either chelator agents (ligands) and their complexes or selected topics of therapeutic interest are shown in tabular form.
Metal chelation principles offer wide new opportunities in the drug design field in addition to the classical answers of metal sequestration or elimination.14‒18 Ascertain the suitable chemical properties for a given application in ligand design is a hot topic. Synthesizing the appropriate coordination compound, for example, may modify a number of properties including charge, lipophilicity, lability, shape and redox potential. Custom agent chelators are thus at our disposal for testing cellular assays. Finding out whether and how those design elements are translated from the cellular content into the clinical practice is a challenge.
Chelates, among other applications, are used in medicine:5,7,19,20
For example, the most common method of treating heavy metal poisoning is to administer a chelating agent to remove the metal ion. Metal ions such as As, Hg, Pb, Au, form chelates with the thiol groups of enzymes and proteins blocking their functions.21,22 The effect of masses exerted by the chelating agent releases the enzyme from the metal ion, allowing its normal functioning, concentrating the metal chelate in the kidney for urinary excretion. The solubilizing chelating agents of the unithiol or penicillamine type (Figure 1) are the most effective.
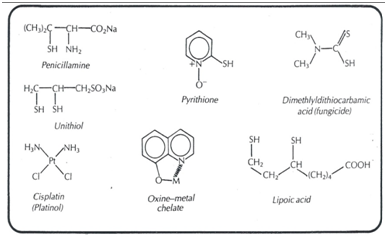
Figure 1 Chemical structure of some ligands of therapeutic interest.20
Contrary to what happens with toxic organic substances, organisms are rarely capable of converting toxic metals into harmless derivatives. Hence, it relies heavily on therapeutic chelating agents to remove toxic metals. On the other hand, the chelate can act as a carrier to release a bioactive ligand. For example, the trans-[PtCl3 (6-mp) 2]2- (Figure 1) transports the cytotoxic ligand 6-mercaptopurine through the membranes thus exhibiting its antitumor activity against cancer.
The fact that only the 8-hydroxy (oxine) member of the series (Figure 1), among the seven isomeric hydroxyquinoline is antibacterial, suggests20 that metal chelation must be involved in its action. In fact, oxine has been used for a long time in the analysis of metals due to its chelating capacity. But is it the antibacterial oxine because it sequesters essential metal ions for the bacterial cell, or is the chelate formed the toxic agent? Apparently, the chelate 1:1 iron-oxine (or the chelate 1:1 copper-oxine for anti-fungal action) is the bactericide, since both oxine and Fe (II) alone kill staphylococci; neither is the iron-oxine chelate 1:2 the active agent. Other chelating agents, such as pyrithione and dimethyldithiocarbamic acid23 have a unique bioactivity (Figure 1).20 Possibly, metal chelates oxidize lipoic acid, the essential coenzyme for the decarboxylation of pyruvic acid.
Inorganic elements embrace those that exert a natural (either beneficial or harmful) biological effect, and other used with medicinal purposes as drugs or probes.18 Elements widely used such as iron, copper and zinc, are included as beneficial members of the first group, as well as others, e.g. cadmium and tungsten, used for only a few species. Nature24 has selected through evolution those elements:
Most of the metal cations present in biological systems exist as chelates or coordination complexes, and monitoring the interaction of these metals with cellular components is a complex task.18 For example, calcium plays a role in enamel and toothpaste, iron in blood and muscle function, molybdenum in the metabolism of purine, zinc in insulin and in various enzymatic systems, chromium in the metabolism of glucose, and magnesium in the transmission of nerve impulses.20 In Figure 2 the periodic biomedical table is observed, figuring the elements with different colours associated with its class or function.25

Figure 2 The biochemical periodic table.25
The practice of medicinal inorganic chemistry goes back to 5000 years ago.26‒29 The use of copper for the sterilization of water by the Egyptians dates back to 3000 years ago. The Arabian and Chinese used gold in varying medicines 3500 years ago (more because of its prized nature than for its proper medicinal action). The discovery of zinc as a promoter of wound healing occurred about 1500 years ago, a time when various iron-based remedies were already applied in ancient Egypt.
The biomedical inorganic chemistry (Table 1),30 (Figure 3)19,31 is a new important area of chemistry and offers potential for the design of new therapeutic and diagnostic agents,8,15,32,33 and therefore, for the handling and the understanding of disorders that lack treatment. Metal ions, including metalloenzymes, activate or biotransform a pleyade of organic compounds used in medicinal practice, that as such compounds do not exert such an action. Organic compounds may also have an effect, either direct or indirect, on the metabolism of the metal ion.30

Figure 3 Some of the areas of medicinal inorganic chemistry.34
No |
Issue |
Content |
1 |
The Biomedical Periodic Table |
Essential elements |
2 |
Metal based therapeutic agents |
Anticancer drugs |
3 |
Metal based diagnostic tools |
Radiopharmaceuticals |
4 |
Biological targets for metal based therapies |
Metalloenzyme inhibition |
5 |
The future of bio-inorganic chemistry |
Polyoxometallates in medicine |
Table 1 Topics to be covered by biomedical inorganic chemistry30
The functions of metal ions27,31,35 in biology are shown in Table 2,30 and their importance is shown in Table 3.30 Medicinal chemistry36 allows exploiting a wide range of reactivities based on the different coordination number and geometries available,28 the varying oxidation states, the thermodynamics and kinetics features, Table 4,30 and the inherent properties of the metal cations and the individual ligands.
Metal |
Function |
Typical deficiency symptoms |
Na,K |
Charge carrier, osmotic balance |
Death |
Mg |
Structure, hydrolase, isomerase |
Muscle cramps |
Ca |
Structure, trigger, charge carrier |
Retarded skeletal growth |
V |
Nitrogen fixation, oxidase |
N/A |
Cr |
Glucose intolerance |
Diabetes symptoms |
Mo |
N2 fixation, oxidase, oxo transfer |
Retardation of cell growth |
Mn |
Photosynthesis, oxidase, structure |
Infertility, impaired growth |
Co |
Oxidase, carbon group transfer |
Pernicious anaemia |
Fe |
O2 transport and storage, oxidase, electron transfer, N2 fixation |
Anaemia, disorders of the immune system |
Ni |
Hydrogenase, hydrolase |
Growth depression dermatitis |
Cu |
E-transfer, O2 transport, oxidase |
Artery weakness, liver disorders |
Zn |
Structure, hydrolase, male fertility |
Skin damage, stunted growth, retarded sexual maturation, impaired development |
Se,As |
Puberty and grown |
Impaired development |
Table 2 Functions of metal ions in biology30
Topic |
Kind |
Catalysing reactions via |
Hydrolytic |
Stabilizing structure |
Protein |
Charge balancing |
Osmotic balance |
Replication and information encoding |
|
Table 3 Why are metal ions important in biology?30
Aspects |
Effects |
Factors influencing |
Kinetics |
Ligand Exchange rates |
Oxidation state |
Thermodynamics |
Complex stability and formation constant |
Oxidation state |
Table 4 Chemical considerations30
Advantage can also be taken of the properties of biologically exotic elements, e.g., the transition elements of the first and second row, and the lanthanides. Gadolinium, a dark lanthanide element, is located in the middle of the Periodic Table. Due to their (unique) characteristics magnetic properties, gadolinium has come to occupy a place in medical diagnosis in the course of a couple of decades. Gadolinium (III) is at the centre of MRI (magnetic resonance image), one of the spectacular advances in medicine,37 in a similar way to platinum in cancer therapy, or technetium in cardiac scanning. A special issue of Accounts of Chemical Research containing reviews on molecular image chemistry was published in 2009.
The successful case of cisplatin in the treatment of various kinds of neoplasms has positioned metal-based drug coordination chemistry at the forefront of the fight against cancer.38 Limit dose side effects or the appearance of acquired resistance are the limiting factor in the treatment with cisplatin. In this sense, the introduction of new Pt drugs has modified the situation, at least partially. This led to the development of carboplatin and other platinum-based cytotoxic drugs. Unfortunately, some platinum-based drugs developed as a result of shortcomings emanating from the clinical use of cisplatin were also associated with severe side effects that had prevented regulatory authorities from granting their marketing approval.39 Similarly, gold (III) complexes have been found to exhibit toxic effects; the most adverse cases of gold complex toxicity are restricted to skin and mucous membrane as reported in case of blind clinical trial.40 In a related development, increased ceruloplasmin and copper levels in various tissues have been associated with cancer progression.41 Most of these adverse effects are dose related and can be circumvented by structural modification of the metal-based complexes to enhance selectivity and reduce unwanted effects on normal cells.42‒45 The search of solutions to these problems has encouraged a intense research in order to give a response to the urgent necessity of developing alternative strategies directed to different targets, by using varying metals, allowing improving the pharmacological features. The use of nanoparticles provide an advanced bioavailability, in vivo stability, intestinal absorption, solubility, sustained and targeted delivery and therapeutic effectiveness of several anticancer drugs generating a big challenge in the dosing of metal-based complexes in cancer therapy.42 Therefore, the opportunity provided by nanoparticles to selectively target cancer cells has gained interest in the design of metal-based cytotoxic drugs.
The use of chelating agents to combat the toxicity of metals and metalloids dates back to the beginning of the last century (Alfred Werner, Paul Ehrlich, Carl Voeftin). The aim was decreasing the arsenic and antimony-containing drugs toxicity used in the treatment of parasitic maladies such as syphilis, trypanosomiasis and schistosomiasis.46,47 Low molecular weight chelating agents were applied in order to alleviate the effect of heavy metal and metalloid accumulation. The use of chelating agents to alleviate fortuitous exposures to metal ions begins in 1941 with the (questionable) use of citrate for lead poisoning.
The most evident medicinal application of chelating agents is the handling of metal accumulation cases.21,2,48‒55 This use has been fcentred on the complexation and elimination of metals from the human body, such as Pb, Hg, Sb and As. Given the characteristics of these metals, most of the first ligands used for this purpose (Figure 4)46 have sulphur as donor atoms.
2,3-Dimercaptopropanol known as British anti-Lewisite (BAL) dates from 1940. It is an antidote to Lewisite (dichlorovinylase), although it was never used as a war weapon. The first use of BAL was associated with arsenical therapies against syphilis and industrial accidents with arsenic. BAL, less toxic, more hydrophilic analogues: meso-2,3-dimercaptosuccinic acid (DMSA), and DL-2,3-dimercapro-1-propanesulfonic acid (DMPS) appear later, being registered in some countries for use in mercurial poisoning treatment.
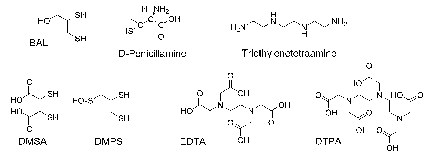
Figure 4 Pro-ligands used to treat accumulation and cases of intoxication.46
The need of using lipophilic BAL painful intramuscular injections, led to the obtainment of an orally active copper chelating agent, (2S)-2-amino-3-methyl-3-sulfanyl-butanoic acid or D-penicillamine (D-pen). Ethylenediaminetetraacetic acid (EDTA), introduced in 1950 for the lead poisoning and for accidental cases involving radionuclides, was the next used in clinical practice. EDTA was substituted later by diethylenetriamine pentacetic acid (DTPA) mainly for the removal of radionuclides. The introduction of triethylenetetramine for patients with Wilson's disease intolerant to D-penicillamine dates from the 1980s. Although its power is less, it is an efficient substitute to the D-pen and for some experts the first choice. Deferoxamine has been the standard chelating agent most used in the treatment of iron accumulation since 1970; the derivative pyridone deferiprone (L1), and the tridentazo deferasirox agent (ICL 670) found oral use for the same purpose in the 80s and 90s, respectively.
Figure 5 shows the chelating agents approved (clinically verified) for the FDA in case of metals overload. The species being subject of removing may be a native metal, such as Fe or Cu, o a contaminant reaching the body in a fortuitous or accidental way, such as As, Hg or Pu. Chelate therapy function implies to stimulate the inhibition of transgressing metals, by first sequestering them in form of high affinity complex, which is excreted then through the liver or kidney.21,22
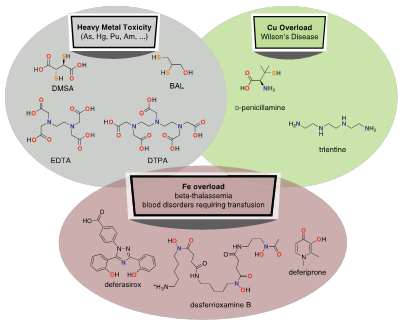
Figure 5 Chelating agents approved by the FDA for indications related to heavy metal toxicity, copper accumulation and iron accumulation. DMSA (meso, 2,3-dimercaptosuccinic); BAL (dimercaprol, meso-2,3-dimercaprol); EDTA (ethylenediaminetetraacetic acid); DTPA (diethylenetriaminepentaacetic acid); o-penicillamine (Cuprimine, Dpen, dPen); trientine (triethylenetetramine, trien; desderrioxamia B (DFO, deferoxamine, desferal); deferasirox (Exjade); and deferiprone (Ferriprox).
The classical therapy chelation notion involves the use of a chemical agent, administered with the purpose of removing a metal from the organism.21,22,54 However metal chelate formation can lead to biological implications that go beyond the simple elimination of the metal.16 The exploration of these possibilities can lead to therapeutically decisions (interventions) that vary the concentration, distribution, or reactivity of metals to achieve beneficial effects.
Four general strategies find use16 in medicinal contexts beyond the usual concept of chelation therapy (Figure 6A):

Figure 6 Chelation use strategies in the medicinal context.16
In recent decades, the hypothesis has been deepened that chemically defined chelating agents may be potentially useful in the treatment of 6,55‒57 neurodegenerative diseases. The moderate success of the bidentate chelating antimicrobial agent clioquinol has contributed to an increase in their use. Although its use in Alzheimer’s disease started from the classic idea of sequestering agent, the mechanism of biological action is much more complex.58‒60 It has been observed “in vitro” that Cu2+ and Zn2+ raise the disintegration speed of beta amyloid (A) peptide, preventing compounds such as clioquinol this process, even “in vivo”. Clioquinol (Figure 7A),16 acts as an ionophore that may transport ions through biological membranes.
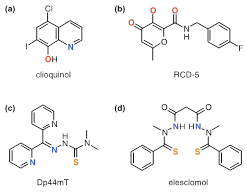
Figure 7 Special chelating agents mentioned in the text.16
Well-designed inhibitors using chelating agents allow occupying the metal coordination positions blocking access to the substrate and thus interrupting the catalytic functions. RCD-5 inhibits HIV integrase by binding to the active position of the enzyme di-magnesium, via chelating 3-oxygens (Figure 7B).16 The rapid proliferation of cancer cells demands iron, so chelation on this way can be an efficient anticancer strategy. However, the biological effects of iron potent anticancer chelating agents with potent anticancer activity are complex, and linked to its redox activity, not with iron depletion. The chelating agent Dp44mT (Figure 7C),16 with potent antitumor activity, is an interesting example. The elesclomol (Figure 7D),16 evaluated in clinical trials, seems to work in an analogous way to mechanism related with copper. On the other hand, a masking group can first block the binding position realising then the chelating unit by an activation means.16
Next, a brief description of main ion metal complexes having medicinal interest is presented. Metal ions play important roles in biological processes, and the field of knowledge concerned with the application of inorganic chemistry to therapy or diagnosis of disease is medicinal inorganic chemistry.11 Platinum, gold, iron, copper and aluminium are considered in this context.
Platinum
In the 1960s it was found that cis-diaminodichloroplatinum (II), or cisplatin, inhibits the cell division of Esterichia Coli leading to the ensuing discovery of the effective antitumor capacity in mouse models of this simple coordination compound.34,38,61,62 Cisplatin was validated later in humans, approving the FDA its use for the treatment of testicular and ovarian cancer in 1978. Its introduction in the therapeutically arsenal improves the survival prospects of many cancer patients. The cure of testicular cancer with modern platinum chemotherapy went from less than 10% before its introduction, to 90%. Cisplatin kills cancer cells by cross-linking with the DNA strand and inhibition of transcription phenomenon. Despite the curative success with this kind of cancer, cisplatin is not universally effective against other types of cancer provoking a number of side effects. In addition, certain types of cancer resist to therapy with cisplatin. The resistance may be either intrinsic or it may appears after a prolonged treatment. To overcome these difficulties, new platinum complexes have been devised and their anti-tumour properties investigated.11,12,38,63 Although thousands of compounds have been prepared and tested, very few are approved for clinical use (Figure 8).38
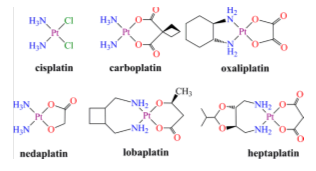
Figure 8 Chemical structures of anticancer drugs for clinical use based on platinum. The complexes of the upper part are approved all over the world; those at the bottom are approved in Japan, China, and Korea, respectively.38
Pt (IV) complexes enter the medical scenario shortly after cisplatin toxicity became a major clinical problem.61 It is believed that the octahedral complexes of Pt (IV) are kinetically more inert in the bloodstream, but can be activated by reducing agents once they penetrate the cells, originating cytotoxic species of Pt (II). They have the advantage with respect to the compounds of Pt (II), of oral availability and reduced resistance and toxicity. They offer an advantage over Pt (II) compounds such as oral availability and reduced resistance and toxicity.
Thousands of Pt (IV) complexes synthesized have been evaluated in the context of prodrugs. The two best-known examples are satraplatin (JM216) and LA-12. The satraplatin (Figure 9),61 is in an advanced clinical trial for prostate, lung and ovarian cancer; it is activated in the presence of intracellular reducing agents such as glutathione and ascorbic acid, among others, and shows little neurotoxicity, neurotoxicity and cytotoxicity. LA-12 (Figure 9),61 is an analogue of satraplatin containing a bulky hydrophobic ligand, adamantylamine, which can potentially increase the release of LA-12 by cancer cells, being efficient against ovarian carcinomas resistant to cisplatin, in a significative way.
BBR 3464 (Figure 9),61 a charged polynuclear complex, is more potent than cisplatin and shows good activity against resistant cell lines. Clinical trials (the only proven compound not based on the cisplatin chemotype) began in June 1998 and are currently progressing in Phase III (treatment of patients with melanoma, pancreas, lung, ovary, and gastric tumours). The complex consists of two monofunctional Pt moieties and forms wide-range cross-linked adducts. Multinuclear complexes of Pt (II) offer different reactivities and biological activities than cisplatin and analogues.61 Ruthenium has several oxidation states (2+, 3+ and 4+) that can coexist under physiological conditions. The ruthenium complexes have the same kinetic spectrum of substitution of ligands as the Pt (II) complexes in aqueous medium, being therefore appropriate alternatives to the anti-cancer drugs of Pt (II). In recent decades, the activity of various ruthenium complexes (2+ and 3+) has been synthesized and studied against several model tumours, but only real progress is made with the arrival of NAMI-A and KP 1019, currently under clinical trials These complexes are particularly useful in the treatment of metaplastic tumours resistant to cisplatin.61
Gold

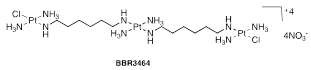
Figure 9 (A) JM216 (Satraplatin); (B) LA-12; y BBR3464.54
Among the non-Pt anti-tumour agents, gold complexes have received in the last decades an increasing attention due to their great inhibitory effect on the growth of tumour cells, being able to exploit their pharmacodynamics and pharmacokinetics properties and their mechanisms of action.34 In recent decades, new compounds of Au (I) and Au (III) with different molecular structures have been developed and tested as anticancer agents. Gold complexes with phosphine, carbene, diethyldithiocarbamate ligands, and porphyrinates have been the most investigated, “in vitro” and “in vivo”. Although its mechanisms of action have yet to be elucidated, some relevant biochemical properties of gold complexes are common. For example, the inhibition of thioredoxin reductase, binding to protein and DNA, the triggering of anti mitochondrial effects and the induction of apoptotic events, among others, have been confirmed for different gold compounds, which contribute to their pharmacological profiles.61
Gold complexes such as auronofin (Figure 10),19,46 formulated for oral use, unlike their injectable precursors, e.g. gold sodium thiomalate, are administered in the treatment of rheumatoid arthritis. Although the mechanisms of the therapeutic (and toxicological) effects are still uncertain, it is thought that auranofin carries out immunosuppression by a variety of actions. The gold compounds were first applied47 in the treatment of rheumatoid arthritis in 1927, and are still applied in the treatment of severe cases.
Iron
Oxygen is transported in the blood by haemoglobin, an iron-containing protein, which is composed of two subunits called α and β. The β-thalassemia is a genetic disorder that affects more than 100,000 children each year, consisting of the fact that haemoglobin units are not synthesized in adequate amounts, which translates into anaemia.64‒67 Children affected by the disease survive only with the frequent transfusion of red blood cells. The result of continuous blood transfusions is the toxic accumulation of iron in the body.
The total iron in the body of the healthy man is maintained at a constant level of about 4-5g, the daily absorption (1-3mg) being balanced with the average daily loss in an equivalent amount. A severe iron deficiency in the diet can affect the synthesis of haemoglobin resulting in anaemia, but in most cases it is easily treated with iron supplements. Serious toxic effects that are more difficult to treat arise as a result of an excess of iron in the body, which is known as iron overload. Some thalassemic patients can accumulate 40 to 50g of iron in a period of 10 years.66 This is a threat to life since iron, by virtue of its easy redox chemistry, is toxic when it occurs in excess, lacking the organism lacks mechanisms of excretion of large quantities of it.
The (intensive) therapy consists in the treatment with chelating agents to clean the organism of excess accumulated toxic iron, having successfully used desferrioxamine B, a powerful chelating agent of Fe3+, produced by Streptomyces pilosus. This molecule is quite selective for iron, showing much lower affinities for copper, zinc, calcium and magnesium.66 Given its high solubility in water, it is capable of selectively removing iron and facilitating its excretion by bile and urine. However, it has the disadvantage of being inactive orally and only works well when administered subcutaneously or intravenously in periods of 8-12 hours up to six times a week. This makes it difficult to comply with the treatment. Hence the intense search for active chelating agents by mouth.
The obvious method of choice when designing new selective iron chelating agents is to model compounds based on natural siderophile structures. Like all living cells, microorganisms require iron as an essential nutrient, being this metal a cofactor of numerous enzymes and respiratory proteins. Siderophores are compounds of low molecular weight (500 to 1000 Da) with very high affinities towards iron. The coordination of the metal occurs in most cases via oxygen atoms, either from the hydroxamate or catechol groups. However, these functional groups have disadvantages for their clinical application.
The hydroxamates are sensitive to the acidic environment of the stomach, some being subjected to breakage catalyzed by enzyme, while the catechols are rapidly oxidized in the intestine and also poorly absorbed. Attempts to overcome these difficulties led to the design of non-hydrolysable analogues of the natural siderophore enterobactin, but many of its derivatives are insoluble in water. Although this latter limitation can be overcome by introducing sulphonate groups, the absorption of the resulting high charge derivatives is then very low. An additional drawback of the tricatechol type ligands is that they lead to the formation of charged complexes of iron (III), trapping the metal intracellularly and thus preventing its excretion.
Traditionally, polycarboxylic ligands have been used to chelate transition metals, including iron (III). However, these chelators are not specific enough for iron, and their use in the body in chelation therapy can lead to the elimination of other essential metals, for example calcium and zinc. This is why a systematic search has been undertaken for molecules that possess the characteristic selectivity for iron of hydroxamates and catechols, and that lack their disadvantages.66 In particular, neutral ligands are searched, e.g. hydroxypyridones, which give rise to neutral iron complexes, which are absorbed in the gastrointestinal tract and are capable of penetrating the cells, at the same time that the resulting neutral complex freely exudes from them. Clinically, useful iron chelating agents should be able to get iron not bound to proteins, but not with iron bound to enzymes, redox proteins and oxygen carriers, since they could interfere with cell function in their normal case and induce an ample spectrum of undesirable side effects. Desferrioxamine reduces the incidence of heart and liver diseases in patients with thalassemia. In those in which iron accumulation is effectively controlled, 91% survival is free from heart disease after 15 years of treatment.67 Many potent iron-chelating agents have been tried, although very few have been introduced into clinical practice. Deferiprone (Figure 5), introduced in 1987, has been used with positive effects in more than 50 countries (but its use is not allowed in Canada and the United States). The structure and activity of deferiprone and other 3-hydroxy-4-pyridones used in chelation therapy against iron accumulation has been studied by computational and optical methods.68 Specific iron chelating agents (CSI) can be used in the healing of neurodegenerative processes such as Alzheimer's, Parkinson's and prion diseases, in which iron plays a significant role. The combined use of desferroxyamine and deferiprone increases survival and reduces heart disease in relation to the isolated use of each of them. In 2005, a chelating agent for oral use, deferasirox (Figure 5), was approved in the United States. No current treatment is totally effective; hence the interest that this field of work awakens (Table 1). In the long term, bone marrow transplantation or gene therapy could cure the disease.
Copper
Copper is an essential element for life; many enzymes contain copper.69 Copper acts as a catalytic and structural cofactor, and consequently intervening on this way in a variety of biological routes. Since copper is necessary for the activity of these enzymes involved in normal metabolism, and is not synthesized by any biochemical process, its inclusion as a dietary component is essential. For these reasons, much attention has been given to the investigation of its mechanisms of absorption, distribution, metabolism and excretion. Attention has also been paid to clarify its function in the development of cancer and other diseases. The bioavailability of copper is depending70 on varying factors, fundamentally:
The fundamental aspects of copper chemistry and biochemistry, the role of copper in medicine, the pathology and treatment of Menken and Wilson's diseases, and chelation therapy in neurodegenerative disorders involving their accumulation have been treated with detail41,71,72 in recent reviews. The readers may consult these sources to obtain an in-depth understanding of the multifaceted roles that copper ion plays in physiology.
The concentration of copper in the human body is closely regulated at the level of cells, organs and body, since free copper ions are potentially detrimental. Once copper is absorbed in the stomach and small intestine, its distribution in the bloodstream is regulated in the liver by ceruloplasmin and albumin. Complex control mechanisms transport copper then through cell membranes fundamentally via copper transporter protein (CTR1) during import and Cu ATP7A/B during export.36 All these proteins have many residues (mainly methionine, histidine and cysteine) to which copper is co-ordinated.
The copper complexes in particular are remarkable active pharmacological agents, activity that we can compare with that of the isolated copper ions or that of the free ligands. An increase of the compounds containing copper in the blood plasma, amino acids and albumin and ceruloplasmin complexes is observed as part of a general physiological response to disease states such as infections, arthritis, epilepsy and cancer. Copper may reaches a concentration of 1.67mg/L in the serum of patients with breast cancer, concentration greater than 0.98 mg/L, the normal reference. When the disease is overcome, copper levels return to normal.
Wilson's disease, i.e. hepatolenticular degeneration, is a metabolic disorder featured by copper accumulation in different organs, yielding liver cirrhosis, neurological disturbances and a complexity of symptoms related to the mismatch of the transport and distribution of copper throughout the organism (frequently affected patients have low levels of ceruloplasmin) in serum. Different chelating agents have been used in clinical practice in therapy.70
Copper complexation is a strategy that can be used for tumour reversion; tumour cells constitute a proper, selective target for an antitumor drug. The donor ligand atom is of vital worth as it may modulate:
The stability towards transchelation reactions with physiological molecules (individual amino acids, peculiar peptide sequences, or proteins) should also be subject of consideration in designing copper complexes. Many of them have been proposed as promising cytotoxic agents on the basis of in vitro tests. However very little data concerning with their mechanisms of action have been the subject of publication.
Currently, the control of angiogenesis, tumour growth, and metastasis can be achieved by chelating the copper excess.36 Small molecules with the ability to bind copper have been synthesized and structurally manipulated (Figure 5), e.g. trientine (trien), D-penicillamine (D-pen) and tetrathiomolybdate (TM). Though copper chelators have been exploited as potential therapeutic agents for some types of cancers, their clinical approval have been usually circumscribed to patients with heavy metal poisoning or diseases with a severe metal accumulation (Wilson's disease). It has recently been shown that mixtures of copper salts with dithiocarbamates (DTC) or clionicol (CQ) spontaneously bind with the cell tumour copper forming a proteosome inhibitor and an inducer of apoptosis. The penicillamine complexes with Cu (II) (benzylpenicillin, phenoxymethylene-penicillin, ampicillin, amoxicillin, and carbanicilin) show stoichiometries of type 1:1, 1:2 and 1:3. The structure of the compounds behaves as bidentate ligands coordinated by the Cu (II) ion through the carboxyl groups. The copper (II) ions promote the hydrolysis of penicillin to the corresponding penicilloic acids, due to the β-lactam group. This same process28 has been confirmed for Cu (II) ions and carboxyl groups of cefazolin, which also belongs to the family of lactam-antibiotics. Bisthiosemicarbazone.56,73‒77
Aluminium
Aluminium compounds find a wide use in the food, textile, dye and paper industry, as well as in cosmetic and pharmaceutical preparations, and in human and veterinary medicine.78 A buffered aspirin with aluminium glycinate, for example, has use as an analgesic. The atmospheric acidification is a progressive cause of a massive transport via acid rain of aluminium from the mountains (mineral deposits) to the surfaces of the waters, exposing plant, animal and man species, to interact with cationic absorbable Al species.79,80 Aluminium products were widely used in all human activities until the 70's, date in which was considered as origin of different diseases, especially osteodystrophy and dementia in patients with kidney failure under dialysis treatment. The knowledge of the toxicity of Al led to a substantial rising in the chemical and biochemical research of this metal ion.81The hydrolytic chemistry of aluminium greatly affects its solubility and bioavailability in biological media. The speciation of the equilibria of the different forms of aluminium hydroxocomplexes, either soluble or insoluble, together with other competitive ligand complexes formed, must be taken into account to explain its solution in biological systems.79‒81
The accumulation of aluminium in the body is not common, but it can come from physiological disorders that can give rise to an excess of aluminium compared to its slow natural process of elimination. Today the syndrome of dialytic encephalopathy is less common due to the absence of aluminium in dialysis equipment. The accumulation of aluminium is also of interest because excessive amounts of it are present in patients suffering from Alzheimer (the causes are not clear and may be a mere side effect). Desferriferrioxamine-B (desferal, DFO),80 a microbial siderophile that has been widely used in the treatment of Cooley's anaemia and other forms of iron accumulation, is recommended for the treatment of Al(III) accumulation. The pyridone L1 also has a high affinity for aluminium and can also be used to remove excess aluminium in the human body.
The following is a tabular summary of the contents of works related to the use of ligands and complexes of therapeutic interest, paying attention to the most relevant and current bibliography. Table 5 is ordered on the basis of the most frequently studied metal ions. Table 6 contains a miscellany of topics based on selected key words, of interest in the subject that concerns us.
Metal |
Comment |
|
Pt |
Metallointerlators based on the use of Platinum82 |
|
Anticancer agents based on Platinum83 |
||
New platinum compounds based on bidentate ligands O, S84 |
||
Synthetic methods for the preparation of anticancer platinum complexes38 |
||
Anticancer compounds based on platinum85 |
||
Distribution of Platinum drugs in cancer cells and tumours86 |
||
From platinum to gold-dithiocarbamate complexes in cancer therapy34 |
||
Current status of antitumor drugs based on Platinum87 |
||
Pt (NH3)2Cl2 and cancer88 |
||
Au |
Prospects for the future of organometallic gold complexes89 |
|
Therapeutic agents based on gold90 |
||
Fe |
Fe brain therapy by iron chelation of deferasirox-lactoferrin conjugates57 |
|
Iron chelating agents in photodynamic therapy: synergistic effect of active thiosemicarbazones91 |
||
Chelating agents for the systemic treatment of iron accumulation92 |
||
Design of iron chelating agents with therapeutic applications93 |
||
Designs of macromolecular iron chelating agents of clinical utility94 |
||
Iron chelating agents in the treatment of iron accumulation95 |
||
Chelating properties of new 3-hydroxypyridin-4-one96 |
||
Iron bound to non-transferrin and iron-citrate complexes in thalassemic serum97 |
||
Chelating agents in the treatment of disorders due to iron accumulation98 |
||
Toxicity and modes of action and future possibilities of iron chelating agents99 |
||
Chelation of iron under physiological conditions100 |
||
Chelating agents of iron in clinical practice101 |
||
Iron chelation therapy66 |
||
Iron: nutrient and poison102 |
||
Iron chelation therapy20 |
||
Thiosemicarbazone and pyrazinylhydrazone of picolinaldehyde in iron accumulation103 |
||
Chelation and metabolism of iron104 |
||
Al |
Chelating agents in aluminium accumulation78 |
|
Perspectives in the chelation of aluminium and iron in the brain105 |
||
In vitro and in vivo studies of hydroxypyridone complexes with aluminium106 |
||
Speciation of aluminium in human serum79 |
||
Study models for aluminium and Alzheimer disease107 |
||
Coordination of Al (III) in the environment and in biological systems80 |
||
Cu |
Control of redox potential and lipophilicity with Cu(II)-bisthiosemicarbazone complexes as PET radiopharmaceuticals73 |
|
Redox properties of therapeutics Cu(II)-bisthiosemicarbazone complexes75 |
||
Cu(II)-bisthiosemicarbazone complexes as antibacterial agents74 |
||
The multiple facets of copper in medicine and treatment71 |
||
Copper complexes as anticancer agents36 |
||
Biochemical aspects of copper and its complexes in medicine41 |
||
Copper related diseases70 |
||
Copper-doxorubicin in cancer therapy108 |
||
Dual behaviour of ionic copper in biology72 |
||
Copper complexes as anticancer agents109 |
||
Copper-thiomolybdate complexes in Wilson's disease110 |
||
Copper complexes in Biochemistry and Pharmacology69 |
||
Gd |
Chelates of Gd (III) as contrast agents111 |
|
Gadolinium complexes as contrast agents112 |
||
New contrast agents MRI113 |
||
Complexes of Gd (III) as contrast agents MRI37 |
||
Pb |
Chelation therapy in lead poisoning: 3,3-dimercaptosuccinic acid114 |
|
Hg |
Rational design of chelating agents for mercury115 |
|
Hg/Cd |
Chelation of Mercury and Cadmium; speciation, 2,3-Dimercaptosuccinic acid116 |
|
Chelation therapy in intoxication by these metals117 |
||
Te |
Tellurium poisoning: scientific coincidence118 |
|
Re(VII) Tc(VII) |
Trioxo complexes of Re (VII) and Tc (VII) stabilized by tridentate ligands as radiopharmaceuticals119 |
|
Zn |
New zinc complexes with heterocyclic ligands as antimicrobial agents120 |
|
Bi |
Chelating nitrogen rich macrocyclic ligands of therapeutic bismuth radioisotopes121 |
|
Bioinorganic chemistry of bismuth and antimony: metallodrugs122 |
||
Ti |
Bioinorganic chemistry of titanium24 |
|
Co(II) |
Cobalt compounds of multifaceted chemotherapeutic agents123 |
|
Cobalt derivatives as therapeutic agents124 |
||
Vitamin B12, (cyanocobalamin): electronic structure125 |
||
Pu |
Activity and rational design of sequestering agents126 |
|
Chelation "in vivo", mixed octodentate ligands based on spermine127 |
||
Lanthanides |
Complexes in leishmaniasis or Chagas disease128 |
|
Lanthanide complexes in osteoporosis129 |
||
Lanthanide compounds in bone resorption disorders130 |
||
Therapeutic applications of lanthanides131 |
||
Therapeutic agents radiopharmaceutical132 |
Table 5 Selected reviews on applications of ligands and / or complexes of therapeutic interest
Topic |
Comment |
|
Alzheimer |
Bioinorganic Chemistry of Alzheimer Disease133 |
|
Chelating agents in Alzheimer’s disease58 |
||
Metal chelating properties of thioflavin-based intercalation compounds59 |
||
Coordinating capacity of metal for compounds containing multifunctional carbohydrates60 |
||
Cancer |
Advances and future perspectives of metal complexes as anticancer agents63 |
|
Update from drug design perspective of metal complexes in cancer therapy11 |
||
Cardiovascular |
Rationale and data in the chelation therapy on cardiovascular diseases134 |
|
Clarifying the role of the treatment with EDTA for prevention of cardiovascular disease135 |
||
Effect of chelation therapy on cardiovascular disease136 |
||
Malaria |
Synthesis of four different metal complexes with potential malaria activity10 |
|
Neurodegenera-tive diseases |
Bis(thiosemicarbazone) metal complexes as therapeutic agents in neurodegenerative diseases6 |
|
Parkinson’s disease |
Mechanisms and biochemical processes in connection with metals and Parkinson’s disease9 |
|
AEDT |
Interaction AEDT-renal stones137 |
|
Ligands in food, cleaning and photography138 |
||
Ligands in Medicine and Personal Hygiene139 |
||
Oxidation |
Oxidation active iron chelating packaging material140 |
|
Lipid oxidation, chelating agents, antioxidants141 |
||
Iron chelating polyethylene film142 |
||
Chemical methods to evaluate antioxidant capacity143 |
||
Supra |
Supra Biomedical applications of complexes with macrocyclic ligands144 |
|
Supramolecular metal complexes as anticancer agents145 |
||
Supramolecular Approach to Medicinal Chemistry146 |
||
Ligand Design |
Design of ligands in Medicinal Inorganic Chemistry15 |
|
Guiding principles in the design of ligands147 |
||
Design of ligands in Medicinal Inorganic Chemistry148 |
||
Multifunctional |
Current trends and future directions of multifunctional ligands in Medicinal Inorganic Chemistry149 |
|
Metal complex |
Radiometals in radiopharmaceuticals150 |
|
Potential of organometallic complexes in Medicinal Chemistry151 |
||
Organometallic compounds as anticancer agents152 |
||
New metal complexes in cancer therapy153 |
||
Natural products as anticancer agents154 |
||
Metal complexes with anticancer activity155 |
||
New agents in the therapy of tropical diseases, immunodeficiency virus156 |
||
Radiometallic agents in cancer therapy157 |
||
Metal complexes: theory and diagnosis of drug resistance158 |
||
History, current status and perspectives of anti-tumour agents other than platinum159 |
||
Toxic metals |
New strategies in the separation of toxic metals from soil and water160 |
|
Environment |
Chelating agents in the environment161 |
|
Difosfonates |
Hygiene products in health care162 |
|
Chemical warfare |
Degradation of chemical warfare agents through reactive polymers163 |
|
Dosing and toxicity |
Summary of in vitro cytotoxic effect of various metal-based compounds with particular reference to their proposed mechanism of action, target and IC50 range11 |
|
Role of copper and the recognition of its complexes as important bioactive compounds in vitro and in vivo as potential drugs for therapeutic intervention in various diseases41 |
||
Platinum anticancer drugs currently approved for use: clinical trials and results39 |
||
Nanoparticles as drug delivery systems in cancer medicine. A challenge in the dosing of metal-based complexes in cancer therapy42 |
||
Toxicity of metal compounds: Basic concepts of toxicity studies and known data are described in a tutorial43 |
||
Clinical trial of dose escalation of lobaplatin in combination with fixed dose docetaxel for the treatment of human solid tumours44 |
||
Combination therapy together with redox modulators to increase the anticancer potency: attractive for lowering the doses of metal complexes that need to be administered45 |
Table 6 Miscellaneous topics related to selected reviews on ligands and / or complexes of therapeutic interest
Chemists and their compounds have contributed tremendously,164 without any doubt, to the impressive progress of medicine. The exploration of the chemistry of coordination offers real possibilities5,8,31,151,164 for a new understanding of intractable diseases and for the design of novel therapeutic and diagnostic agents. The rational design of the chelating agents requires15,19,34 an understanding of link kinetics, catalysis mechanisms and donor interactions. Ligands can be introduced into a biological system to16 limit the adverse effect of the accumulation of a metal ion, to inhibit selected metalloenzymes, or facilitate the redistribution of a metal ion. Some of the mentioned effects involve the modification of reactivity and lipophilicity, stabilization of specific oxidation states or contributions to substitution kinetics. The rational design of efficient chelating agents requires58 a good knowledge of the electronic and molecular structure of the complexes formed.
The discovery (incidental) of the anti-cancer activity of cisplatin in 1960 and its subsequent clinical success generated great interest in the use of metal compounds, Pt and other elements34,61 in the treatment of cancer. The success achieved in this regard by the platinum complexes cisplatin, carboplatin and oxaliplatin has promoted the development of other possible alternatives to be taken into account. Carboplatin is less toxic than cisplatin and can be administered at much higher doses. Oxaliplatin finds use against tumors resistant to cisplatin. Pt (IV) complexes are less toxic than their Pt (II) counterparts and offer many possibilities61 for the rational design of drugs, including oral administration, versus intravenous drugs. Photosensitive Pt (IV) complexes can be activated in tumour tissues to reduce potential damage to healthy people. In addition to the separation of foreign toxic metals and metalloids that do not possess a native biological function, chelation therapy is also used to diminish the essential levels in case of copper and iron accumulation disorders. Given its relative rarity, there is less pressure to develop specific chelating agents86 for the treatment of intoxications. Gadolinium complexes are contrast agents in MRI (about 20 million doses administered per year) and technetium radiopharmaceuticals used in ray image analysis (about 20 million diagnoses per year).35 In recent decades it has been suggested that chelating agents may be potentially useful in the treatment of neurodegenerative diseases or genetic disorders involving dishomeostasis.
The accumulation of iron can come from hemochromatosis, a family of genetic disorders consequence16,64,65 of an unregulated absorption of iron, or of successive blood transfusions necessary to treat blood diseases, ie,-thalassemia and cell disease sickle. Three chelating agents (ligands) have been approved for their treatment: desferrioxamine B, deferasinox and deferiprone. Desferrioxamine is clinically approved19 for the treatment of malaria. Its activity can come from the disruption of iron metabolism in the digestive vacuoles of malaria parasites. In Wilson's disease, the mutation of the ATP7B gene results in the blockage of normal copper traffic,36 leading to an accumulation of copper in the liver that finally spreads to other body organs including the brain. The painful treatment with BAL has given rise to D-penicillamine and triethylene tetramine.
The future of chelating agents designed for therapeutic use in the treatment of diseases evolves to the multifunctional approach.60,83,123,149 In any disease there are multiple biochemical aberrations that must be addressed, and although combination or cocktails of different therapeutic agents could be used for this purpose, a more desirable option is to integrate all these functionalities into a single molecule. For many centuries, the use of metallodrugs has been promoted by empiricism.26 While random screening is still a useful means of drug discovery, today these steps are guided by rational design. The tailor-made design of metal-based drugs to treat specific diseases and disorders is likely to be an important15,35 of the future of personalized medicine, which will include the genomic and proteomic profile of individuals.
None.
The authors declare that there is no conflict of interest

©2018 Martin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.