eISSN: 2379-6367


Research Article Volume 5 Issue 2
Department of Pharmacy, University of Asia Pacific, Bangladesh
Correspondence: Mohammad Shahriar, Department of Pharmacy, University of Asia Pacific, Bangladesh , Tel 881841844259
Received: January 30, 2017 | Published: April 17, 2017
Citation: Das S, Akhter R, Huque S, et al. In vitro anthelmintic activity of leaf extracts of four different types of calamus species. Pharm Pharmacol Int J. 2017;5(2):72-76. DOI: 10.15406/ppij.2017.05.00118
Development of anthelmintic resistance and high cost of conventional anthelmintic drugs led to the evaluation of medicinal plants as an alternative source of anthelmintics. In the present study, methanol, ethanol and chloroform leaf extract of Calamus guruba, Calamus viminalis, Calamus erectus and Calamus tenuis were explored for anthelmintic activity at two concentrations (50 and 100mg/ml), using adult earth worm Pheretima posthuma. All the leaf extracts of Calamus species tested for the anthelmintic activity possess significant activity in a dose dependent manner as compared to the albendazole. The overall findings of the present study have shown that Calamus guruba, Calamus viminalis, Calamus erectus and Calamus tenuis contain possible anthelmintic compounds.
Keywords: Calamus guruba, Calamus viminalis, Calamus erectus, Calamus tenuis,Anthelmintic activity, Pheretima Posthuma, Albendazole, In vitro study, hookworm infestation, entrobiasis, filariasis, taeniasis; hydatidcyst, fluke infection, helminthiasis,wonder drug, niclosamide, oxyclozanide, bithionol
Infections caused by various species of parasitic worms (helminths) of the gastrointestinal tract are the most widespread of all chronic infections of humans in developing countries including Bangladesh and produce a global burden of disease that exceeds better-known conditions, including malaria and tuberculosis.1 It is estimated that approximately one-third of approximately three billion people that live on less than two US dollars per day in developing regions of sub-Saharan Africa, Asia, and the Americas are infected with one or more helminth.2,3 The common helminthic infections in Bangladesh are ascaiasis, hookworm infestation, entrobiasis, filariasis, taeniasis, hydatidcyst and fluke infection (liver, blood, intestinal tract). The current antihelminthic therapies act by incapacitating the parasite by paralysis, damaging the worm such that the immune system can eliminate it, or by altering its metabolic processes. Because the metabolic requirements of these parasites vary greatly from one species to another, drugs that are highly effective against one type of worm are ineffective against others and because of the prevalence of helminth infections, treatment of helminthiasis is of practical therapeutic importance although the synthetic drugs used in helminthiasis treatment have some potential side effects.4 The present experimental work was designed for the search of anthelmintic activity of medicinal plants. Extracts of four medicinal plants of Arecaceae family were conducted in this study. Calamus guruba is well known in Bangladesh as chikan bet or jali bet. The fruits of C. guruba are the most commonly used parts of this plantand are widely used in traditional medicine and food. The fruit is traditionally known as a tranquilizer and a general “wonder drug.” It is a sedative, hypotensive, muscle relaxant as well as it’s used to cure cough, cold, pulmonary disorders. In addition, the fruit extract have demonstrated some enzymatic antioxidant activities. Study on the nutritional composition of the fruits of C. guruba demonstrated the presence of total (1.73%) sugar, (1.15%) protein, (6.45%) carbohydrates and (72.39%) moisture content.5 Biological study of this plant is yet to be conducted. Calamus viminalis is known as Khorkoijja bet in Bangladesh and is widely used as handicraft and furniture material. Ripe fruit pulps are edible. This plant has also been used in traditional medicine for treatment of dog bite, urogenital and gynecological infection.6 The leaf extract of C. viminalis demonstrated to have gastrointestinal motility, antipyretic activity, anti-nociceptive activity and Neurophramacological activity with no potential acute toxicity.6 Calamus erectus is used for making huts and poultry houses. The seeds of this plant are widely used as chewable food. Antihyperglycemic effect of methanol extract was found to be more effective.7 Very few extensive biological studies have been carried out extensive biological study on this plant. Calamus tenuis Roxb is used for making high quality handicrafts. The cultivation of this species for the production of edible shoots is becoming increasingly widespread in Lao PDR, and also North-east Thailand as the shoot is edible. Phytochemical screening of the fruits of C. tenuis reveals the presence of alkaloid, tannin, flavonoid, and steroid which may be responsible to have antioxidant and cytotoxic potential.8 The leaf extract of C. tenuis demonstrated to have gastrointestinal motility, antipyretic activity, anti-nociceptive activity, Neurophramacological activity and anti-diarrheal activity.9 The present study was performed for the first time and the objective of the study was to determine in vitro anthelmintic activity of methanol, ethanol and chloroform leaf extracts of C. guruba, C. viminalis, C. erectus & C. tenuis.
Collection, identification and processing of plant samples
Fresh leaves of C. guruba, C. viminalis, C. erectus & C. tenuis were collected from Botanical Garden, Mirpur-1, Dhaka in November 2015 and all the four plant samples were taxnomically identified with the help of the National Herbarium of Bangladesh, Mirpur-1, Dhaka, where each voucher specimen was deposited. These plants were referenced under the following number: 42754/ DACB, 42755/ DACB, 42756/ DACB and 42757/ DACB respectively for C. guruba, C. viminalis, C. erectus & C. tenuis. The leaves were washed thoroughly 2-3 times with running tap water and were sun dried for seven days in order to remove the moisture contents and then ground into coarse powder using high capacity grinding machine (Jaipan designer mixer grinder, jaipan, India) which was then stored in air-tight container with necessary markings for identification and kept in cool, dark and dry place for the investigation.
Extraction procedure
The powdered plant parts (30 gm) were successively extracted in a soxhlet extractor at elevated temperature (40-60°C) using 300 ml of distilled methanol followed by ethanol, and chloroform. After extraction all extracts were kept in refrigerator at 4°C for future investigations with their necessary markings for identification.
Experimental animal
For the experiment, earthworms were collected from moist soil, near the area of The Board of Intermediate and Secondary Education, Comilla and washed with normal saline to remove soil and fecal matter during the experiment. The earthworms of 5-7 cm length and 0.2-0.3 cm width were used for the experimental protocol.
Preliminary Phytochemical Screening
Different extracts were subjected to preliminary phytochemical screenings to determine the nature of phytoconstituents by using standard protocols.10
Anthelmintic activity
The anthelmintic assay was carried as per the method of Ajaiyeoba et al.11 with minor modifications. In this experiment, Pheretima posthuma were used because of its anatomical and physiological similarity with intestinal roundworm parasites of human beings and for the fact that they belong to same group of Annelida. All the test solutions and standard drug solutions were prepared freshly before starting the experiment. Albendazole (10 mg/ml) was used as reference standard while saline water served as a control. One hundred and fifty six earthworms were divided into twenty six groups with equal size & each group containing six worms. 60 ml formulations containing two different concentrations of methanol, ethanol and chloroform extracts of C. guruba, C. viminalis, C. erectus & C. tenuis leaves (50 and 100 mg/ml in distilled water) were prepared. The time for paralysis (in min) was noted when no movement of any sort could be observed except when the worms were shaken vigorously. The time of death of the worms (in min) was recorded after ascertaining that worms neither moved when shaken vigorously or when dipped in warm water (50°C).
Statistical analysis
All the data are was expressed as Mean ± SEM (Standard error of Mean).The results were analyzed statistically by ANOVA followed by Dunnet’s test. Results below *p<0.05, **p<0.01 and ***p<0.001 are considered statistically significant.
Table 1 represents the results of the phytochemical screenings of the leaf extracts of of C. tenius, C. viminalis, C. guruba, and C. erectus.
Name Of The Test(S) |
C. Tenius |
C. Viminalis |
C. Erectus |
C. Guruba |
|||||||||
Methanol |
Ethanol |
Chloroform |
Methanol |
Ethanol |
Chloroform |
Methanol |
Ethanol |
Chloroform |
Methanol |
Ethanol |
Chloroform |
||
Alkaloid |
Hager’s test |
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
_ |
++ |
+ |
_ |
Mayer’s test |
++ |
+ |
- |
+ |
++ |
- |
+ |
_ |
_ |
+ |
_ |
_ |
|
Wagner’s test |
++ |
++ |
- |
+ |
+ |
- |
+ |
+ |
_ |
_ |
_ |
_ |
|
Carbohydrate |
Molisch’s test |
- |
+ |
- |
- |
+ |
+ |
+ |
++ |
_ |
_ |
+ |
_ |
Fehling’s test |
++ |
- |
+ |
- |
+ |
+ |
_ |
+ |
_ |
_ |
+ |
_ |
|
Flavonoid |
Lead acetate test |
- |
+ |
- |
++ |
+ |
- |
++ |
++ |
_ |
+ |
+ |
_ |
Ferric chloride test |
+ |
+ |
- |
++ |
- |
- |
++ |
+ |
_ |
+ |
+ |
_ |
|
Steroid
|
Libermann Burchard’s Test |
- |
+ |
- |
++ |
_ |
_ |
+ |
++ |
_ |
++ |
_ |
+ |
Saponin |
Froth Test |
+ |
+ |
+ |
_ |
++ |
++ |
++ |
_ |
_ |
++ |
++ |
+ |
Phenol |
Ferric chloride test |
+ |
+ |
+ |
+ |
+ |
- |
+ |
++ |
_ |
+ |
+ |
+ |
Tannin |
|
+ |
+ |
- |
+ |
+ |
- |
++ |
+ |
+ |
++ |
_ |
|
Glycoside |
|
+ |
+ |
- |
++ |
+ |
- |
_ |
+ |
_ |
++ |
_ |
_ |
Fixed oil |
|
++ |
++ |
- |
_ |
+ |
+ |
+ |
_ |
+ |
+ |
+ |
_ |
Table 1 Phytochemical screening for the leaf extracts of C. tenius, C. viminalis, C. guruba, and C. erectus
[(+): Presence, (++): Strong presence, (-): Absence]
In vitro anthelmintic activity was conducted with methanol, ethanol and chloroform leaf extracts of C. guruba, C. viminalis, C. erectus & C. tenuis with the doses of 50 mg/ml and 100 mg/ml. The result of the anthelmintic activity of methanolic leaf extracts of C. guruba, C. viminalis, C. erectus & C. tenuis as well as reference drug are presented in Figure 1 & Table 2. The methanolic leaf extracts of C. viminalis, C. erectus & C. tenuis demonstrated death of worms in less time as compared to albendazole especially at higher concentration of 100 mg/ml. Methanolic extracts of selected Calamaus species produce dose dependent paralysis ranging from loss of motility to loss of response to external stimuli in a significant manner (**p<0.01; ***p<0.001) which gradually lead to death. Among the four selected plant methanol extract of C. tenuis produce a significant decrease in paralysis time (**p<0.01) and death time (***p<0.001) as compared to the standard albendazole.
|
|
SS |
df |
MS |
F |
P |
Paralysis Time |
Between |
7145.926 |
8 |
893.241 |
106.338 |
0.000 |
Within |
378 |
45 |
8.4 |
|
|
|
Total |
7523.926 |
53 |
|
|
|
|
Death Time |
Between |
8729.815 |
8 |
1091.227 |
84.0363 |
0.000 |
Within |
584.333 |
45 |
12.985 |
|
|
|
Total |
9314.148 |
53 |
|
|
|
Table 2 Comparative statistical variance of analysis (ANOVA) on anthelmintic activity of methanol extract (leaf) of C. guruba, C. viminalis, C. erectus & C. tenius
*SS: Sum of Squares; df: Degree of Freedom; MS: Mean Square; F: Fisher value; P: Probability value 0.05. Each value represents mean ±SEM (N=6). Data are found to be significant by testing through one way ANOVA with replication at 1% level of significance
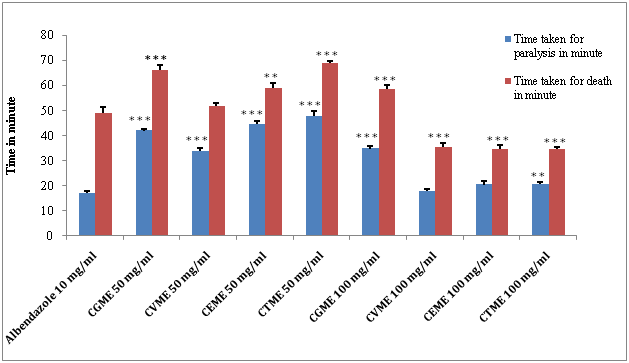
(Methanol extract of Calamus guruba: CGME, Methanol extract of Calamus viminalis: CVME, Methanol extract of Calamus erectus: CEME, Methanol extract of Calamus tenuis: CTME) (Values are expressed as mean ± S.E.M. (n=6), **p<0.01, ***p<0.001significant when compared with the corresponding value of the standard group, done by independent sample test).
The result of the anthelmintic activity of ethanol extracts of C. guruba, C. viminalis, C. erectus & C. tenuis leaf as well as reference drug are presented in Figure 2 & Table 3. The present study with C. guruba, C. viminalis, C. erectus & C. tenuisleaf revealed that ethanol extract (50 and 100 mg/ml) caused paralysis as well as death of worms in a significant dose dependent manner (*p<0.05; **p<0.01; ***p<0.001) . When compared with the activity of ethanol extract of C. guruba, C. erectus & C. tenuis the activity of the ethanol extract of C. viminalis at 100 mg/ml showed significantly less time to cause death (***p<0.001) which is even lower than the standard drug.
|
|
SS |
df |
MS |
F |
P |
Paralysis Time |
Between |
10381.333 |
8 |
1297.667 |
31.725 |
0.000 |
Within |
1840.667 |
45 |
40.904 |
|
|
|
Total |
12222 |
53 |
|
|
|
|
Death Time |
Between |
13669.703 |
8 |
1708.713 |
79.929 |
0.000 |
Within |
962 |
45 |
21.378 |
|
|
|
Total |
14631.703 |
53 |
|
|
|
Table 3 Comparative statistical variance of analysis (ANOVA) on anthelmintic activity of ethanol extract (leaf) of C. guruba, C. viminalis, C. erectus & C. tenius
*SS: Sum of Squares; df: degree of freedom; MS: mean Square; F: Fisher value; P: Probability value 0.05. Each value represents mean ±SEM (N=6). Data are found to be significant by testing through one way ANOVA with replication at 1% level of significance
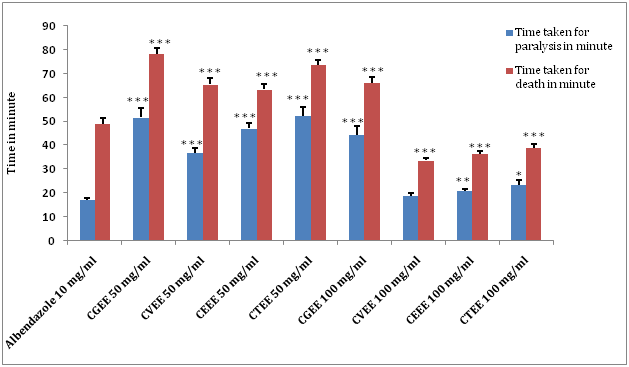
(Ethanol extract of Calamus guruba: CGEE, Ethanol extract of Calamus viminalis: CVEE, Ethanol extract of Calamus erectus: CEEE, Ethanol extract of Calamus tenuis: CTEE) (Values are expressed as mean ± S.E.M. (n=6), *p<0.05, **p<0.01, ***p<0.001significant when compared with the corresponding value of the Standard group, done by independent sample test).
After investigation of chloroform extracts of C. guruba, C. viminalis, C. erectus & C. tenuis leaf, following data were observed (Figure 3) (Table 4), where the higher concentration of chloroform extract (100 mg/ml) with showed significant (*p˂0.05; **p˂0.01; ***p˂0.001) decrease in paralysis and death time. Among the chloroform extracts of all plants, the present study revealed the anthelmintic activity of C. tenius washigher as compared to others, although, the chloroform extracts of C. guruba, C. viminalis, C. erectus & C. tenuisleaf (50 and 100 mg/ml) caused paralysis as well as death of worms in a significant dose dependent manner (*p<0.05; **p<0.01; ***p<0.001).
|
|
SS |
df |
MS |
F |
P |
Paralysis Time |
Between |
6147.148 |
8 |
768.394 |
44.264 |
0.000 |
Within |
781.167 |
45 |
17.359 |
|
|
|
Total |
6928.315 |
53 |
|
|
|
|
Death Time |
Between |
9065.667 |
8 |
1133.208 |
63.970 |
0.000 |
Within |
797.167 |
45 |
17.715 |
|
|
|
Total |
9862.834 |
53 |
|
|
|
Table 4 Comparative statistical variance of analysis (ANOVA) on anthelmintic activity of chloroform extract (leaf) of C. guruba, C. viminalis, C. erectus & C. tenius
*SS: Sum of Squares; df: degree of freedom; MS: Mean Square; F: Fisher value; P: Probability value 0.05. Each value represents mean ±SEM (N=6). Data are found to be significant by testing through one way ANOVA with replication at 1% level of significance.
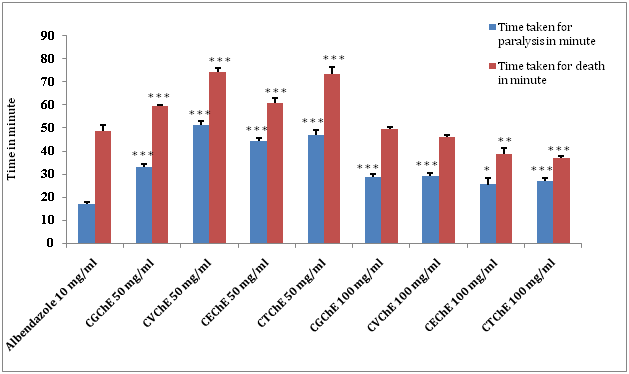
(Chloroform extract of Calamus guruba: CGChE, Chloroform extract of Calamus viminalis: CVChE, Chloroform extract of Calamus erectus: CEChE, Chloroform extract of Calamus tenuis: CTChE) (Values are expressed as mean ± S.E.M. (n=6), *p<0.05, **p<0.01, ***p<0.001 significant when compared with the corresponding value of the Standard group, done by independent sample test).
The use of natural dietary compounds has the potential to be a complementary control option which may reduce this reliance on drug treatment, and slow the development of resistance parasites. In this study, anthelmintic assay of various extracts of C. guruba, C. viminalis, C. erectus & C. tenuiswas performed on adult Bangladeshi earthworms, Pheretima posthuma due to its anatomical and physiological similarity with intestinal roundworm parasites of human beings as well as their ease of availability and have been used widely for the initial evaluation of anthelmintic compounds in vitro. The result of the present study indicates that all the three leaf extracts of Calamus species tested for the anthelmintic activity possess significant activity as compared to the albendazole, which is a broad spectrum anthelmintic. Albendazole acts by inhibiting microtubules polymerization after binding to the colchicine sensitive site of β-tubulin. Which causes decrease in microtubules in the intestinal cells, absorptive function, and uptake of glucose by the adult and larval forms of the parasites thereby depletes glycogen storage. Paralysis and death of susceptible GI parasites occur slowly due to insufficient energy for the production of adenosine trisphosphate and their clearance from the GI tract may not be complete until several days after treatment.4 In addition to its vermicidal properties, it has been found to have both ovicidal and larvicidal activities in human. The use of natural plant extracts as de-wormers for humans and livestock has long been practiced, however scientific validation of these practices and identification of active compounds has been lacking.12-14 Anthelmintic effects of plants are normally ascribed to secondary metabolites such as essential oils,15 flavonoids, alkaloids, terpenoids16 or polyphenols such as proanthocyanidins,17 also known as condensed tannins. Tannins are non-nitrogenous plant constituents and have an astringent action on mucous membranes as they precipitate protein from the cells of mucous membranes and exert a protective action. Some synthetic phenolic anthelmintics e.g. niclosamide, oxyclozanide, bithionol etc., are reported to interfere with energy generation in helminth parasites by uncoupling oxidative phosphorylation.18 Moreover, direct anthelmintic effects of purified condensed tannins have been confirmed in in vitro assays against, amongst others, Haemonchus contortus,19 Ostertagia ostertagi20 and Ascaris suum21 as tannins can bind to free proteins in the gastrointestinal tract of host animal or glycoprotein on the cuticle of the parasite and cause death.4,22 Tannin containing plants increase the supply and absorption of digestible protein by animals.23 This is achieved by the formation of protein complexes in the rumen by tannins, which later dissociate at low pH in the abomasum to release more protein for metabolism in the small intestines of ruminant animals.24
These findings suggest that methanol, ethanol, chloroform extracts of Calamus species have promising anthelmintic effects. We propose that future work should focus on attempting to identify the phytoconstituents for the isolation of pure compounds responsible for the specific biological action as demonstrated in this study.
None.
Author declares that there is no conflict of interest.

©2017 Das, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.