eISSN: 2379-6367


Research Article Volume 4 Issue 4
1School of Pharmacy, BBD University, India
2Proffesor and Principal, Acharya Narendra Dev College of Pharmacy, India
Correspondence: Rajiv Gupta, Professor and Dean School of Pharmacy, BBD University, Lucknow-226 028 India , Tel 91 9839278227
Received: October 30, 2014 | Published: June 7, 2016
Citation: Ranjana, Mishra A, Mishra A, et al. Determination of Gallic acid and β–sitosterol in poly-herbal formulation by HPTLC. Pharm Pharmacol Int J. 2016;4(4):373-379. DOI: 10.15406/ppij.2016.04.00081
Introduction: In recent years, herbal medicines are increasing in popularity all over the world. The use of herbal medicines are being used in both preventative and treatment based usage, as health supplements and tonics because consumers perceive herbals as “natural”, safe, harmless and free from adverse side effects.
Aim: The present studies aims to quantitatively estimate Gallic acid and β-Sitosterol in selected Polyherbal formulations.
Methods and Material: Polyherbal capsules available in market were randomly selected for studies. The Parameters like shape, size, colour, pH, weight variation, Moisture content disintegration time and dissolution time were studied. Chromatographic and HPTLC method was applied for the qualitative as well as quantitative determination of active constituent Gallic acid and β-Sitosterol.
Result: The Gallic acid was found as 9.78µg/ mg and 12.79µg/ mg in S1and S2 respectively and β- sitosterol 4.18µg/ mg and 3.81µg/mg mg in S1and S2 respectively.
Conclusion: It was thought worthwhile to initiate on generation of some data, for the evaluation of such types of Ayurvedic preparation i.e. capsules which may serve as a reference for future studies based on quality control.
Keywords: β-sitosterol, gallic acid, HPTLC, fingerprint, polyherbal, capsules, weight gain
Drugs of Natural origin have been used since ancient times as medicines for treating range of diseases. Medicinal plants have played a key role in world health. In spite of the great advances observed in modern medicine in recent decades, plants still make an important contribution to health care. Each plant is like factory capable of synthesizing unlimited number of highly complex and unusual chemical substances whose structures could otherwise escape the imagination forever.1 The extract of the medicinal plant may consist of several phytochemical constituents, which often act together synergistically/at times antagonistically. Formulations based on natural products have reached widespread acceptance as medicinal agents for plethora of diseases.2
The acceptance or the popularity of herbal products is on the rise, one of the hinderance in its total acceptance is that somewhere the reproducible quality benchmark parameters are lacking, the reasons may be known or unknown , for example due to the highly complex nature and known nature of variation of the phytochemical of drugs of natural origin. Furthermore, the structure elucidation part is at times not fully understood or needs some more studies with reproducible results to reach a decisive conclusion.3
To overcome these uprising problems, standardization of herbal formulations is essential in order to assess the quality of drugs. Herbal medicines may contain up to hundreds of compounds. Although many of them are present in low concentrations, they may be important for the quality, safety and efficacy of the herbal medicines as their therapeutic effects are based on synergetic interactions between numerous constituents. In order to explore the whole profile, chromatography studies combined with a suitable detection technique offers a powerful tool for isolation of the individual phytochemical and thereby developing a characteristic profile of the given plant sample.4
In the traditional text of Indian system of medicine, i.e. Ayurveda, medicinal plants play a significant role in maintaining good health. It would not be wrong to state that there is a resurgence of use of herbal medicines throughout the world and the people are turning back to natural remedies. Herbal medicinal products are dietary supplements that people administer to improve their health and are sold as tablets, capsules, powders, teas, extracts and fresh or dried plants. The literature survey reveals that the many herbs are used in health product provide nutritional supplements help us to overcome the nutritional deficiencies. It also helps us to boost our immune system. Nutritional supplements are also useful in getting rid of the toxins that are accumulated in our body. There are some of plants (Table 1) are commonly used in health products, and also reported in literatures provide as body weight gain.
Sr. No. |
Common Name |
Biological Source |
Effect on Health |
1 |
Ashwagandha |
Withania somnifera |
Anabolic activity of Withania was attributed to the presence of withanolides. Its anabolic effect may be due to the anti-serotonergic activity which would lead to an increase in appetite and therefore weight gain.5,6 |
2 |
Satavari |
Asparagus racemosus |
In Ayurveda it is describe as a ‘rasayana’ herb well known to promote physical and mental health, improve defence mechanisms of the body and promoting physical strength due to “adaptogen effect”.7,8 |
3 |
Neem |
Azadirachta indica |
Due to micro minerals and macro-minerals, It is partially responsible for the effect increase in body weight.9-11 |
4 |
Amla |
Emblica officinalis |
The increase in body weight gain might be due to hepatoprotective activity resulted into improvement in the liver function.12 |
5 |
Lehsun |
Allium sativum |
Reported as, significant increase in body weight of hyperglycemic animals after treatment with herbal preparations containing garlic in hyperglycemic animals.9 |
6 |
Tulsi |
Ocimum sanctum |
The increase in body weight due to Ocimum sanctum treatment might be due to increased immune status resulting in better conversion of feed leading to more weight gain.12 |
Table 1 Commonly herbs used in health supplements
Gallic acid and β- Sitosterol are commonly constituents found in these plants, thus they are active constituent of polyherbal formulation used as health supplements. Gallic acid (GA, 3,4,5-trihydroxybenzoic acid), a plant phenol that occurs naturally and is present in Amla, Haritaki, Bhera , and, tea leaves, grapes, and other plants, both in its free state and as part of the tannin molecule. Gallic acid possesses cytotoxicity against cancer cells, anti-inflammatory, antimutagenic, hepatoprotective, and neuroprotective effect, anti-tumor potential and analgesic activity.13-15
β- Sitosterol is a common phytoconstituents known plant sterol called phytosterols. Its efficacy reported as follows in the literature review. The structures of β- sitosterol and cholesterol are quite similar. The liver function activity (GDP, GOP) can be improved with β- sitosterol, and this can reduce prostate cancer and colon-cancer cell growth too. The presence of β- sitosterol in soybean foods has been reported to inhibit growth of cancer cells. It can also be the factor used to form the lympho cells and NK in the immunity process circulation β- sitosterol can be found in vegetables such as peanut oil. It is used in experiments for treating breast cancer and prostate cancer β- sitosterol in soybean oil has been reported to exhibit hypolipidemic activity.16-18
Thus Gallic acid and β- Sitosterol is important active constituent of many herbal formulations and analysis of these phytoconstituents may exploit the quality of product. This research paper provides qualitative and quantitative determination of these phytoconstituents i.e. Gallic acid and β- sitosterol present in Polyherbal formulation with the fingerprint profile through HPTLC of herbal formulation. In the last two decades HPTLC method has emerged as an important tool for the qualitative and quantitative phytochemical analysis of herbal drugs and formulations.
Collection and Composition of capsules
Polyherbal capsules purchased from local medical shops of two brands (coded as S1 and S2) claiming that weight gain, improve physical and mental health.
Evaluation of quality control parameters for each capsule
Organoleptic parameters: Organoleptic parameters like size, shape, colour of samples were carried out.19
Particle size determination
Particle sizes of capsule content are determined using software Medical Pro version 3.0.
Uniformity of weight: Test for uniformity of weight was performed as per Indian pharmacopoeia, 1996.20
Determination of pH: The procedure given in Indian Pharmacopoeia, published by Govt. of India was followed.20
Determination of Moisture content: Weight about 500 mg of capsule content taken into a weighed crucible, kept in oven at 105-110 °C temperature, cool in a desiccators and weigh after every 15 min. till one gets the constant weight, the loss in weight is usually recorded as moisture.21
Disintegration test for capsule: The procedure given in Indian Pharmacopoeia, published by Govt. of India was followed.20
Dissolution test for capsule: The procedure given in Indian Pharmacopoeia, published by Govt. of India was followed.20
TLC and HPTLC Studies of Capsule content
Solvents and chemicals: Standard Gallic Acid and β-Sitosterol of analytical grade were purchased from S. D. Fine Chem. Ltd (Mumbai, India) and Sigma Aldrich respectively, Methanol, Ethyl Acetate, Acetic Acid (Glacial), Formic Acid, Chloroform and Toluene analytical grade solvents were obtained from S. D. fine chemicals and silica gel GF254 pre-coated TLC aluminum plates purchased from Merck (Mumbai, India).
Preparation of standard solution of Gallic acid: 20 mg of standard Gallic acid accurately weighted and dissolved in 20 ml of methanol to prepare 1000µg/ml stock solution. 2ml, 4ml, 6ml, 8ml and 10ml solution withdrawn from stock solution and added 10 ml methanol of each solution, the prepared dilution are 200µg/ml, 400µg/ml, 600µg/ml, 800µg/ml, and 1000µg/ml.
Preparation of standard solution of β- Sitosterol: 20 mg of standard β-Sitosterol accurately weighted and dissolved in 20 ml of methanol to prepare 1000µg/ml stock solution. 2ml, 4ml, 6ml, 8ml and 10ml solution withdrawn from stock solution and added 10 ml methanol of each solution, the prepared dilution are 200µg/ml, 400µg/ml, 600µg/ml, 800µg/ml, and 1000µg/ml.
Preparation of test solution: The 100 mg capsules content dissolved in 100 ml of methanol in beaker and continuously shaking for 4 hrs in magnetic stirrer and filter. This filtrates used for TLC and HPTLC studies of Gallic acid and β-Sitosterol, and the same procedure applied for second sample.
Instrumentation
A Camag HPTLC system equipped with a sample applicator Linomat IV, Camag twin plate development chamber, TLC (Thin Layer chromatography) Scanner III and win CATS software used.
Chromatographic condition: Chromatography was performed on 10x10 cm aluminum backed TLC plate coated with 0.2 mm layer of silica gel, application of std. and test sample was done. Spotting was done at 10 mm distance on the TLC plate, ascending development of the plate, was performed with optimized solvent Toluene: ethyl acetate: formic acid (5:5:1 v/v) for Gallic acid and Toluene: Ethyl acetate: Glacial acetic acid (6:2:0.1) solvent used for β-Sitosterol as mobile phase in a camag chambers are previously saturated for 60 min. The average development time was 15 minutes. After development the plate was air dried 5 minutes. Densitometric scanning was then performed with winCATS software at λmax = 254 nm for Gallic acid and 273 nm for β-Sitosterol using Deuterium light source, the slit dimensions were 5.00 X 0.45 mm.
Quality control parameters
The polyherbal supplements were tested for their organoleptic parameters like shape, size, colour and basic quality control parameters that included test like pH, weight variation, Moisture content disintegration time and dissolution time. These parameters are very important as they directly influence the body when consumed. The results are tabulated in Table 2. The standard calibration curve for dissolution time is shown in Figure 1.
Parameters |
S1 |
S2 |
Size |
0 |
0 |
Shape |
Oblong |
Oblong |
Colour |
Dark red |
Green |
Weight variation |
505.75±4.88 mg |
509±3.83mg |
pH |
6.46 |
6.55 |
Moisture content |
6.40% |
6.30% |
Disintegration time |
10 min. |
12 min. 46 sec. |
Dissolution time (after 50 min). (fig. 1) |
16.01% |
12.22% |
Particle size (Average circle diameter) (fig.2) |
105.72 micron |
100.53 micron |
Table 2 Quality Control Parameters
Thin layer chromatography
Each sample solution with standard was spotted on the TLC plate, ascending development of the plate, was performed with optimized solvent Toluene: ethyl acetate: formic acid (5:5:1 v/v) for Gallic acid and detection was done by keeping plate in Iodine vapour chamber and Toluene: Ethyl acetate: Glacial acetic acid (6:2:0.1) and detection was done by spraying with anisaldehyde - sulphuric acid reagent for β-Sitosterol (Figure 2A) (Figure 2B).
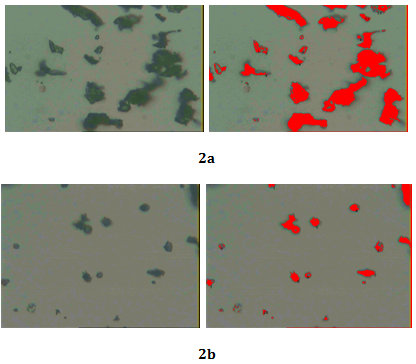
HPTLC studies
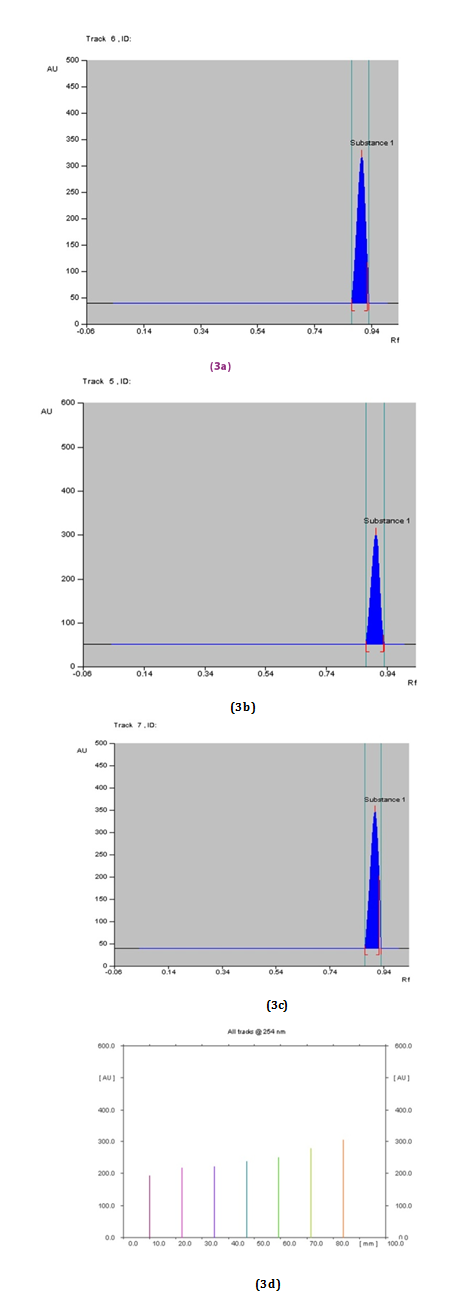
HPTLC of gallic acid: Ascending development of the pre coated TLC plate, was performed with optimized solvent Toluene: ethyl acetate: formic acid (5:5:1 v/v) mobile phase for detection of Gallic acid (Figure 3A-3D). Densitometric scanning was then performed winCATS software at λmax = 254 nm. For using Deuterium light source, the slit dimensions were 5.00 X 0.45 mm Table 3.
Tracks |
Concentrations of Standard Gallic Acid (in μg/ml) |
Max. RF |
Area under curve (AUC) |
Track 1 std. |
200 |
0.91 |
5267.2 |
Track 2 std. |
400 |
0.91 |
5584.5 |
Track 3 std. |
600 |
0.91 |
5683.4 |
Track 4 std. |
800 |
0.91 |
6042.8 |
Track 5 std. |
1000 |
0.91 |
6317.3 |
Table 3 RF and area under curve values for different concentrations of working standards of Gallic acid for linear calibration at 254 nm
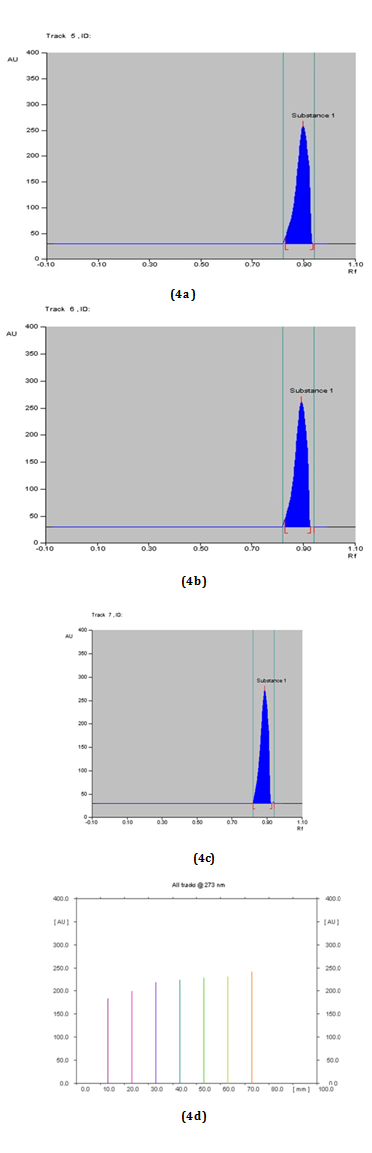
HPTLC of - β-Sitosterol: Ascending development of the precoated TLC plate, was performed with optimized solvent Toluene: Ethyl acetate: Glacial acetic acid (6:2:0.1) solvent used for β-Sitosterol as mobile phase (Figure 4A-4D). Densitometric scanning was then performed with a Camag TLC Scanner 3 equipped with winCATS software at λmax = 273 nm (Table 4).
Tracks |
Concentrations of Std. β-Sitosterol (in μg/ml) |
Max. RF |
Area Under Curve (AUC) |
Track 1 std. |
200 |
0.92 |
7383.5 |
Track 2 std. |
400 |
0.92 |
8658.3 |
Track 3 std. |
600 |
0.92 |
9575.6 |
Track 4 std. |
800 |
0.91 |
9874.3 |
Track 5 std. |
1000 |
0.9 |
10178.2 |
Table 4 RF and Area under curve values for different concentrations of working standards of β-Sitosterol for linear calibration at 273 nm
Validation of HPTLC method21-24
Linearity: Concentration range observed was 200–1000μg. The peak area (y) is proportional to the concentration of Gallic acid (x) following the regression equation y= 255.8x+5011. The calibration plot showed the correlation coefficient (r2 = 0.979), intercept was (5011) and slope was (255.8) over the concentration range (Figure 5), and in the concentration range of 200-1000μg/ml the peak area (y), and the concentration of β- Sitosterol (x) following the regression equation y =680.5x+7092. The calibration plot showed the correlation coefficient (r2 = 0.903), intercept was (7092) and slope was (255.8) over the concentration range (Figure 6).
Sensitivity: The sensitivity of measurement of Gallic acid and β- Sitosterol by the use of proposed method was estimated in the terms of Limit of detection (LOD) and Limit of quantitation (LOQ). The LOD and LOQ were calculated by the use of equation LOD = 3.3 x σ/S and LOQ = 10 x σ/S, where S is slope of calibration curve and σ is the standard deviation of the peak areas of the standards. The LOD and LOQ for Gallic acid found to be 4.71µg/ml and 14.29µg/ml respectively (Table 5) and the LOD and LOQ for β- Sitosterol found to be 4.90µg/ml and 14.87µg/ml respectively.
Parameters |
Results |
Linear range (n=5) |
200-1000µg/ml |
regression equation |
y =255.8x+5011 |
Correlation coefficient (r2) |
0.979 |
Slope (m) |
255.8 |
Intercept |
5011 |
Limit of detection (LOD) |
4.71 µg/ml |
Limit of quantitation (LOQ) |
14.29 µg/ml |
Conc.(x) Of Gallic acid in sample S1 |
9.78 µg/mg |
Conc.(x) Of Gallic acid in sample S2 |
12.79 µg/mg |
Table 5 Validation parameters for the estimation of Gallic acid by the proposed HPTLC Method
Specificity: The specificity of the method was ascertained by analyzing standard and sample. The spot for Gallic acid and β- Sitosterol in sample was confirmed by comparing the Rf and spectra of the spot with that of standard (Figure 4,5). The peak purity of Gallic acid and β- Sitosterol was assessed by comparing the spectra at three different levels, i.e. peak start, peak apex and peak end position of the spot (Figure 7,8).
Fingerprint of herbal formulation
A fingerprint of an Herbal formulation is a chromatogram here representing all the detectable chemical components present in the extract and being separated as much as possible so as to identify and characterize that Herbal Medicine. With the help of the fingerprint, the authentication and identification of Herbal Medicine can be reliably conducted even if the quality and quantity of the constituents are unknown.4
Fingerprint of S1 and S2 shows (Figure 9A & 9B) the maximum peaks in optimized solvent i.e. Chloroform: ethyl acetate: Methanol (6: 2: 2 v/v) as a mobile phase.
It has been proven that from the days of antiquity medicinal plants have been used by traditional/ folklore therapy for various prophylactic/therapeutic uses. Therefore it is now need of the hour that the multi component herbal formulations can be standardized with newer techniques such as High performance thin layer chromatography, for reproducible results with quality benchmark parameters. HPTLC is the most significant method which can be used for routine herbal drug analysis and for quality assurance.
The poly herbal formulations were evaluated on the basis of organoleptic and physiological parameters. Data thus generated suggested that each capsule and its extract were consistent with various identity, quality and purity parameters such as organoleptic parameters, physic-chemical parameters, and HPTLC analysis. Capsules passed the test for uniformity of weight. Capsules disintegrated within 30 min. Dissolution of capsules were 12%-17%. Moisture content of capsules was more than 5%, which indicates that there are less chances of degradation.
A chromatographic method was developed for separation of Gallic acid, using Toluene: ethyl acetate: formic acid (5:5:1 v/v). Similarly another chromatographic method was developed for separation of β-Sitosterol using Toluene: Ethyl acetate: Glacial acetic acid (6:2:0.1) as a mobile phase. The proposed HPTLC method was found to be rapid, simple and accurate for quantitative estimation of gallic acid and β-Sitosterol present in both samples. The Amount of Gallic acid found as 9.78 µg/mg and 12.79 µg/mg in S1and S2 respectively and β- sitosterol 4.18 µg/mg and 3.81 µg/mg mg in S1and S2 respectively. Multiple peaks were observed in the chromatogram of poly herbal formulation samples extracted from capsules, which can be used for routine polyherbal drug analysis and for quality assurance.
The authors are thankful to Prof. (Dr.) Shubhini A Saraf for providing the necessary laboratory facilities and AICTE-MODROBS Grant (F. No.8024/RID/BOR/MOD-458/2009-10), for making the research work possible.
Author declares that there is no conflict of interest.

©2016 Ranjana,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.