MOJ
eISSN: 2379-6294


Review Article Volume 2 Issue 1
Department of Agricultural Development, Democritus University of Thrace, Greece
Correspondence: George Vassiliou, Department of Agricultural Development, Democritus University of Thrace, Greece, Tel 3069 4606 4664
Received: November 24, 2015 | Published: February 22, 2016
Citation: Vassiliou G. Factors affecting risk assessment of pesticides in water bodies: a review. MOJ Toxicol. 2016;2(1):26–32. DOI: 10.15406/mojt.2016.02.00030
Pesticides pose a significant threat to the environment in terms of toxic effects. However pesticides toxicity is shaped by exposure factors and ecosystem characteristics. Such factors determine whether and in what extent the pesticide’s toxic potential will be actually expressed. Evaluation of the impact of such factors upon the pesticides toxic potential in a given environment is the task of risk assessment. The present study reviews the factors affecting the source, application pattern, load and availability of pesticides to water bodies, determining in this way the exposure status. Further on new trends in toxicity testing e.g. tiered and tailor made methodologies as well as the enhancement of the predictive principle in pesticides risk assessment are highlighted. Finally deterministic and probabilistic approaches in pesticide risk characterization are discussed.
Keywords: pesticide, toxicity, exposure, risk assessment
ERA, ecological risk assessment; GAP, good agricultural practice; EFSA, european food safety authority; ARfD, acute reference dose; USEPA, united states environmental protection agency; Koc, adsorption coefficient; MECs, measured environmental concentrations; LD50, lethal dose; PNEC, predicted no effect concentration; RQ, risk quotient; LOC, level of concern
The potential of a chemical substance to cause one or more adverse effects to biological systems defines its toxicity. Effects, in terms of quality and quantity are supposed to be identical under standard conditions (toxicity assays and tests under strictly defined protocols). These effects could be expressed in different life organization levels, e.g., molecular, biochemical, physiological or even behavioral levels. Yet, under different ecosystems the potency of these effects depends on factors like exposure duration, intensity and distribution, organisms and hence population susceptibility, vulnerability of biota concerned, temporal or/and spatial emission of the toxic agent as well as nature of the emissions sources (point- or non-point sources). Thus different ecosystems exhibit different vulnerability to contaminants due to their dependence on factors mentioned above. Hence a case per case study of the probability for a contaminant to demonstrate unacceptable adverse ecological effects upon a defined ecosystem, e.g. ecological risk assessment has grown in significance the last ten years. Ecological risk assessment process is accordingly based on exposure data in abiotic or/and biotic elements of a certain ecosystem as well as on effects caused by such an exposure upon the ecosystem.
Pesticides are chemical substances that are deliberately applied mainly in agro ecosystems but reach natural ecosystems through precipitation, drift and runoff. Due to often unavoidable interactions between natural ecosystems and human activities (Figure 1) they pose a profound risk to public health (Figure 1).
In this case pesticides function as primary stress factors to ecosystems which in turn act as secondary stressors to humans. However since uncertainty about the outcome is always present assessment of the evolving risk should be conducted in most cases. Pesticide residues derive from widespread point (commercial sites) as well as non point sources and cannot be controlled at any step after their application. Moreover as they appear in mixtures their potency as well as their toxicity is often underestimated (synergistic effects). Pesticide active ingredients are either dissolved in water bodies (hydrophilic substances) or are particle bound (potential for particle bound transport). Due to this fact pesticide residues transport in aquatic ecosystems does not follow the normal distribution. Therefore pesticide Ecological Risk Assessment (ERA) though fully justified is often complicated and a case per se.
ERA of pesticide residues in surface waters basically comprises assessment of factors affecting exposure, adverse effects (toxicity) and ecosystem characteristics.1 Ecosystem itself (study object of Eco toxicology) and not just single organisms (study object of Environmental Toxicology) is the ultimate investigation object of ERA.
The present review aims to
Water bodies are reservoirs having water the whole year or at least over a long period of the year. Consequently they include surface waters as well as groundwater bodies. It is obvious that risk assessment due to pesticide residues is applicable to both categories referring either to the respective ecosystems elements or to humans since both surface as well as ground water bodies serve as water drinking sources. Pesticides reach water bodies through drift, runoff or drain flow. Actually drift is the most studied way of contamination of water bodies.2 ERA of a pesticide is actually the assessment of the probability for the expression of its toxic potential upon ecosystems. Basic questions to be answered are: a. which are the factors to be considered in order to assess this probability with a decent certainty and b. Quantification of this probability itself.3 The answer to first question refers to the substance availability in the relevant environmental departments which generally follows a two-phase process.4 Source, initial concentration in water, route of the substance, spatial and temporal distribution and finally available concentrations constitute the exposure characteristics of a pesticide active substance in a defined ecosystem. Recently simulation models predicting the substance distribution in the ecosystem under study have been developed.5 Main factors affecting the pesticides availability to biological systems and thus shaping the expression of their toxic potential are as follows:
Application scheme of pesticides in agro ecosystems
Any factor (dose / concentration, application frequency, label restrictions oversight) leading to deviations from Good Agricultural Practice (GAP) could enhance their toxic expression in aquatic ecosystems. Special constituents of the application scheme are following factors:
Single or multi-application scheme: Repeated applications can have a different final risk assessment result than a single application. Repeated or pulsed application schemes may increase the selection pressure for tolerant individuals resulting to a different assessment decision compared to a single application assessment.6 Herbicides are as a rule applied once, while insecticides and especially fungicides are often applied repeatedly. This is a rule especially for fungicides applied preventively. An ecosystem has usually the ability to recover from a pesticide stress factor. Repeated exposures to pesticide reduce the chance for the ecosystem to recover.7
Direct application or indirect exposure: Direct pesticide application on water surfaces is conducted for vector control, while indirect exposure takes place mainly through runoff, drift or precipitation. In the first case pesticide loads are significantly bigger than indirect exposure.
Buffer zones between site applied and bystanders: Although guidelines for setting up and calculating buffer zones when applying pesticides (especially fumigants) are now quite strict8 this is not always the case in some countries. This fact increases the probability of contamination of adjacent zones. In all cases proximity of the application site to the adjacent water body should be taken into account.
Fate of the parent compound in the environment
The pesticide parent active ingredient is normally degraded in water in less hydrophobic and as a rule less toxic transformation products. Yet, these products alone or combined are in no case to be considered as non toxic. Such product can be produced not only through the natural environmental processes but also through water treatment processes such as water purification and disinfection. Therefore approaches have been developed for assessing ecological risk.9 Risk assessment processes of pesticide metabolites in human diet have lately developed to a rather significant matter of discussion in European Food Safety Authority (EFSA).10 The Agency discussed possibilities of applying the concept of Threshold of Toxicological Concern and proposed assessment schemes for chronic and acute dietary risk of pesticide metabolites, using the TTC approach. This approach has as ultimate target to define generic threshold levels for human health for all chemicals and is utilizing in a way the Acute Reference Dose (ARfD) approach.11
Volatility of the substance
Only very few pesticides exhibit volatility over 10-6 mm Hg, the limit over which pesticides are considered as hazardous air pollutants.12
Solubility in water
Water soluble substances are easily and very effectively transported in water. US Environmental Protection Agency (USEPA) sets the water solubility value of 3 mg/L as the limit over which a substance is considered as potential water pollutant. Yet, this is not to be considered as 100% applicable since substances with water solubility less than 3 mg/L have been found in groundwater.
Adsorption to soil particles
Water soluble substances generally exhibit a reduced potential for soil adsorption. On the contrary non polar (lipophilic substances) bind to soil particles according (among other factors) to their adsorption coefficient (Koc) which is substance (physicochemical properties) and soil properties (organic content, colloidal nature, pH) dependable.
Dissipation of the substance
This is usually expressed through the DT50 of the substance and contributes in combination with the Koc to the leaching potential through the GUS (Groundwater Ubiquity Score) index: GUS = log (DT50) x [4 - log (Koc)]. This is an empirical index and serves only as potential indicator since environmental conditions are not considered. GUS values greater than 2,8 indicate that that leaching of the substance is probable, while this is not likely to happen with GUS values less than 1.8. Intermediate values point to a limited leaching probability. In detail following scheme is applied:13 GUS value and leaching probability: <0.1 extremely low; 0.1-1.0 very low; 1.0-2.0 low; 2.0-3.0 moderate; 3.0-4.0 high; >4.0 very high. However it should be noted that dissipation in groundwater compared to dissipation in surface waters follows a totally different pattern due to different environmental conditions, absence of photolysis and lower hydrolysis rate in comparison to soil surface.14,15 Apart from physicochemical properties dependence pesticide leaching in groundwater is often favored by preferential flow of water and solutes through soil and rock materials.16,17
SCI-Grow index
This index is a screening model used by USEPA18 for assessing pesticide concentrations in vulnerable ground water. It takes into account environmental properties of the substance such as fate, application frequency as well as eventually existing small-scale prospective ground-water monitoring studies. In this aspect it complements the GUS index.
Estimated Environmental Concentrations or Expected Environmental Concentrations are estimates produced by either mathematical models or through a program of monitoring (field sampling) indicating the pesticide concentrations to which non-target areas and consequently biota using them as habitat are exposed. Input information includes pesticide use pattern given in the label as well as data concerning fate of the parent substance in the respective environment. In general, USEPA uses a tiered approach to estimate EECs, using the GENEEC2 as model, which utilizes the worst case scenario estimating EEC in water from sites that are highly vulnerable to runoff or leaching. Other more fine tuned models (e.g., PRZM-EXAMS) are used if higher accuracy is needed.19 In a lot of cases EECs are characterized as Predicted Environmental Concentrations or PEC values. If risk assessment is of short term interest Measured Environmental Concentrations (MECs) e.g., actual concentrations can be used. EECs’ advantage is the long term predictive value, while evolving uncertainty should be properly managed. The EEC of pesticides is then related to the EC50 (concentration causing a 50% reduction in a chosen toxicity endpoint for a given aquatic test organism. At present, if only a few species are involved, the application of an assessment factor is suggested due to intraspecific differences in pesticide sensitivity.
listic approach which should result to assessment of effects to ecosystem and not merely to single organisms. This target is structured to following objectives:
The strategy of methodology used in European Union for pesticides toxicity assessment and characterization is expressed in the new Regulation of the European Parliament (EC N1907/2006) and European Commission (18/12/2006) concerning Registration, Evaluation, Authorization and Restriction (REACH) of chemical substances.20,21
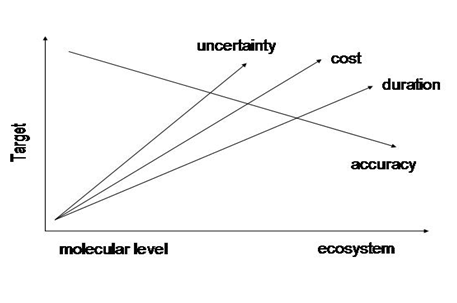
Figure 2 Relation of uncertainty, cost, duration and accuracy of experimentation to life level and level of target completion.
The new strategy determines the number and kind of experiments according to the quantity of the pesticide to be produced, imposes the use of the prediction principle in eco toxicological studies, suggests the use of tiered and tailor made experimentation and transposes the responsibility of implementation upon producers or/and formulators of the substance. As a result of the new strategy predictive value due to modeling reinforcement especially in developmental and genetic toxicity has recently been significantly enhanced. Quantity Structure Activity Relationships (QSARS) e.g., octanol/water partition coefficient (Kow), Bio concentration Factor, sediment/water partition coefficient, molecular volume of the substance, have strengthened significantly the implementation of the prediction principle22 (Figure 2).
Toxicity assessment methods are classified as:
Standardized tests in vivo with single organisms aiming to assess pesticides environmental toxicity evaluate effects like mortality, immobilization, reproduction, growth and fecundity. It should be noted that according to OECD guidelines these tests are conducted provided that the preliminary mortality assay yields an LD50 value less than 100 mg/L.
Tests in vitro usually precede those in vivo and follow the QSARs evaluation. According to European legislation these tests contribute to a better understanding (e.g. mode and mechanisms of action) of the adverse effects and if necessary are being crosschecked with tests in vivo. Most of these tests have not proved to be scientifically valid for pesticides toxicity evaluation. Yet some of them, e.g., Microtox Test, acetyl-cholinesterase inhibition test (organophosphates), aldehyde dehydrogonase inhibition test (atrazine, captafol, carbendazim, diuron, maneb, metolachlor) have been successfully used.23,24
New trends in pesticides toxicity assessment in Europe are expressed by the integrated approach which is envisaged in the REACH regulation. This approach is supposed to contribute to a balance between reduction in experimentation cost and the growing uncertainty. REACH regulation actually imposes a unified toxicity evaluation on the ground of preceded separate assessments of impact upon environment and human health taking into account the predictive values of QSARs. In this sense toxicity evaluation tends more and more to actual risk evaluation.
In USA the integrated approach in pesticide toxicity assessment is also strongly supported by EPA. Thus more assessment endpoints combined with more life stages of organisms in less but better targeted tests are proposed. At the same time the Agency favors a more active involvement of toxicity mechanisms as well as pharmacokinetics in pesticides toxicity assessment.
On the other hand, aiming to a further cost reduction, the use of acute toxicity tests in such a way that toxicity threshold values (normally deriving from chronic toxicity tests) can evolve, is strongly encouraged.25 Economic data are constantly incorporated in the integrated approach by means of new simulation models.26 Yet while using such models, a very high degree of complexity leading to unacceptable uncertainties should be avoided.
Beyond the integrated approach, development and use of alternative toxicity evaluation methods is at stake. This trend known as the 3R (replace-reduce-refine) effort targets the reduction of the number of experimental animals by means of replacement or/and reduction or/and refinement of bio tests.
Risk characterization is practically the ultimate scientific result in the ERA procedure. A successful result implies that following main points have already been addressed.
Acute or chronic risk assessment
It is often crucial to decide whether acute or chronic risk assessment should be conducted. To answer the question nature and load of pesticides, ecosystem characteristics as well as exposure time and frequency have to be taken into account. In surface waters a temporal as well as spatial monitoring scheme is followed by most researchers.27,28 Such a scheme is used even in cases of very lipophilic substances due to potential for particle bound transport. Proximity of the water body to pesticide application site is proportionally correlated to expected load.29 Repeated exposures to pesticides (pulsed applications) which are a usual approach are most often associated with acute effects. Yet for assessing post exposure delayed effects chronic toxicity assessment could be useful. Linearity between concentration and duration of exposure is also a key assessment factor. It has been recorded30 that linear concentration to exposures response curves were achieved with higher concentrations in shorter exposures. Drift or runoff pesticide sources usually result to low concentrations (parts per billion or parts per trillion) in surface waters. In such cases both chronic as well as acute risk assessment could be justified. On the contrary direct vector control treatments would primarily necessitate an acute risk investigation.
Assessment endpoints selection
Final assessment endpoint selection is a crucial factor for the validity of risk characterization. There are three main criteria which lead to the proper choice. Endpoints should be of undisputable relevance to ecosystem under consideration. Ecosystem components analysis is often needed to clarify the entities as well the attributes that are really critical to the specific ecosystem. Susceptibility of the entitities to the stressor(s) under investigation is also a criterion which should be seriously considered. Often there is significant impact of factors like age, duration of exposure and endpoint selection on assay sensitivity.31 To assess pesticide impact on aquatic or mixed ecosystems (aquatic plus terrestrial) the most susceptible species would for example constitute a good assessment endpoint provided they are relative to the ecosystem under study. Crustaceans are very often among the most susceptible species in such ecosystems.32 Species which really represent the ecosystem have to be included in the selection. An additional issue is vulnerability of the entities to be chosen. Entities may be susceptible but for different reasons out of stressors reach. Still choosing the most susceptible and most vulnerable entities would not be the right choice unless relevance to the management goal is absolutely secured. In case of ecological risk assessments that address the ecosystem as a whole, more than one entities need to be included in the endpoints choice. Researchers33 often use organisms representing at least three life levels to obtain threshold LD(C)50 or No Observed Adverse Effects Level/Concentration (NOAEL(C)) values for the most susceptible representatives of the ecosystem under assessment. For populations, communities and ecosystems assessment a methodology which takes into account various interacting components of the system under study has been proposed. Namely the use of approaches like mean strain measurement, state-space analysis, and non metric clustering for analysis of standardized aquatic microcosm data sets has been discussed.34 Apart from enhancing the predictive power of the assessment conclusion, these methods are expected to facilitate the choice of assessment as well as measurement endpoints in such environments. Endpoints also have to fit to the stressors nature. For example herbicide residues in water bodies have primarily to be assessed on aquatic plants since these are the most susceptible among other biota to herbicides. USEPA has identified aquatic eco toxicity benchmarks values concerning fish, aquatic invertebrates, vascular and nonvascular aquatic plants from risk assessments developed for individual pesticides.35 These benchmarks could be among other things useful in the primary phase of risk assessment (problem formulation) where endpoints selection is applicable. On the other hand for complementary identification of certain pesticide group’s selection of specific biomarkers could be useful. Thus fish generally present an excellent tool for testing brain acetylcholinesterase inhibition due to organophosphate contamination.36
However one should not disregard the fact that other non-scientific factors should be considered for the right selection of final endpoints. In many instances sociological, economic and political implications are of undisputed value to the final endpoints selection decision. It is often the case that such factors determine the level of unacceptable risk much more than pure scientific ones.37
Measurement endpoints selection
Measurement endpoints are “Measurable responses to a stressor that are related to the valued characteristic chosen as the assessment endpoints”. Properly selected measurement endpoints are used to quantify risk addressed to the assessment endpoints. Such responses include various yet absolutely measurable attributes of the respective endpoint(s). This can include specific measurements of receptor health, population indices, measurements of exposure, or direct measures of eco toxicological effects.38 Measurement endpoints can measure exposure, effects or ecosystem and receptor characteristics.
According to USEPA Guidelines for Ecological Risk Assessment following definitions are valid:
Referring to pesticides in water bodies, measures of exposure are their actual or estimated concentrations in surface waters or groundwater. Since such residues mostly originate from agricultural use (through drift or runoff) their concentration follows a seasonal application pattern. Therefore in many cases maximum values are used over average or median values, particularly if acute risk assessment is aimed. On the other hand low concentrations are useful for assessing sub lethal effects over time. American Society for Testing and Materials has issued a guide aiming to facilitate endpoints selection.39
Measures of effects are usually critical doses or concentrations which either produce a certain effect on organisms, populations, communities or ecosystems or define a critical threshold of risk. Such measures of effects for aquatic environments (LC50 EC50 and NOAEC values) are practically provided by toxicity studies. A main difference between risk characterization and toxicity characterization is that in risk characterization process these values along with assessment endpoints, exposure and given ecosystem characteristics are used as data input for mathematical models. Although the output values from these models lie within a more realistic range concerning the concrete ecosystem to be assessed compared to values resulting from toxicity assays they are inferior in terms of certainty of results.
Risk Evaluation
According to USEPA40 there are mainly two approaches in evaluating pesticides risk in ecosystems, namely the deterministic and the probabilistic approach.
The deterministic approach uses actually the point “double benchmark concept”. Namely a point measured or estimated concentration of a pesticide in water (usually EEC or PEC) is compared to an aquatic life benchmark. Such benchmarks are usually point estimates of effect concentrations such as LC50, EC50 or NOAEC values corresponding to selected assessment endpoints and assessment targets (e.g., acute or chronic risk).
Regarding acute aquatic risk assessment a certain LC50, value which usually corresponds to the most susceptible organism among the most relevant in the ecosystem under study is taken as benchmark. A chronic aquatic risk assessment requires normally a suitable NOAEC value as benchmark. In cases of lack of scientific evidence for long term toxicity an LC50 value corresponding to the most susceptible representative species and properly extrapolated through an assessment factor (usually 0.1 to 0.001) is applied. This benchmark is the so called Predicted No Effect Concentration (PNEC). The ratio EEC / PNEC is the Risk Quotient (RQ). Concerning the presence of more than one pesticide in a certain aquatic ecosystem USEPA suggests the additive model, the actual RQ being the sum of all substances RQs. This of course is much more valid in cases of pesticides with the same or similar modes of action. Risk Quotients constitute a second benchmark of Level of Concern (LOC). LOCs are threshold values for risk concern set by USEPA for each “risk situation”. Regarding aquatic risk four “risk situations” have been established, namely acute high risk, acute restricted use, acute endangered species and chronic risk with LOCs 0.5, 0.1, 0.05 and 1.0 respectively The deterministic approach has been used extensively for screening purposes of pesticides in surface waters.41-43 Yet it seems that the RQ approach enables incorporation of refined environmental exposures to the classical tier 1 numerical ranking RQ model.44
The probabilistic approach yields a range of values defining also their distribution. It can actually be applied either by incorporating either the entire stressor-response relationship or variability in exposure and/or effects. It is actually a comparison between stressor and exposure response curves. The outcome of such an approach is not merely a low or high risk statement (RQ approach) but an examination of many exposure-effects scenarios with predictive value and certainty calculation. By means of this approach a prediction of the most probable impact upon the ecosystem is made available. This approach can also predict incremental changes in exposures necessary to limit risk to a desired level. Especially for pesticides this method can answer to the question “which would be the appropriate application dose reduction in order to achieve a desirable reduction of risk?” a question which the RQ approach cannot answer. Yet a disadvantage of the method is that secondary effects which might evolve through inconsistencies between available measurement endpoints and ecosystem under study are not considered, unless an appropriate planning has been done in the problem formulation and analysis phases.
Pesticides are chemical substances and as such are likely to express their toxic potential in the environment. This likelihood depends on exposure, toxicity and environmental characteristics. However there are issues as application pattern, selectivity mechanisms, synergistic or antagonistic action and non point source origin which differentiate pesticides from the other industrial chemical substances. These properties should be taken seriously into account when pesticide risk is to be assessed.
New trends in toxicity testing tend to modify the classical approach to a risk assessment procedure. Hence tiered and tailor made testing is recently applied, while QSARs and use of biomarkers are encouraged as complementary measures. Modeling is today a significant tool in pesticide risk assessment with parallel efforts to decrease cost and uncertainty of results.
None.
The author declares no conflict of interest.

©2016 Vassiliou. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.