MOJ
eISSN: 2574-9773


Research Article Volume 1 Issue 6
Department of Physics, Nehru Memorial College, India
Correspondence: Annamalai Rajendran, Department of Physics, Nehru Memorial College (Autonomous), Puthanampatti, Tiruchirappalli, Tamilnadu, India
Received: November 06, 2017 | Published: December 7, 2017
Citation: Senthilkumar S, Rajendran A. Synthesis, characterization and electrical properties of nano metal and metal-oxide doped with conducting polymer composites by in-situ chemical polymerization. MOJ Poly Sci. 2017;1(6):192-195. DOI: 10.15406/mojps.2017.01.00031
The conducting polyaniline (pani) doped with nano aluminium (n-Alpani) and nano aluminium oxide (n-Al2O3pani) composites are prepared by using in situ chemical oxidative polymerization method. The synthesized samples were characterized Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD). The morphological and elemental compositions were analyzed using FESEM with EDX. The electrical conductivity of the pure Pani and its nanocomposites were studied by two probe technique. The very strong bands at 593cm-1 and 615cm-1 corresponds to Al-O stretching vibrations. FT-IR study confirms the presence of aluminium nanoparticles in the Pani matrix. The A.C electrical conductivity of Pani, n-Al2O3pani and n-Al pani were calculated and found to be 8.2 x10-9S/cm, 1.77x10-8S/cm and 2.15x10-6S/cm respectively. The results revealed that there is an increase of one order of conductivity in the metal oxide composite and three order increase of conductivity in the nano metal composite when compared to pure polyaniline.
Keywords: polyaniline, FT-IR, XRD, FESEM(EDX), electrical conductivity
FESEM, field emission scanning electron microscope; PANI, polyaniline; n-Alpani, nano aluminium; XRD, x-ray diffraction; NCs, nanocomposites
In recent years, developments of inorganic–organic hybrid materials on nanometer scale have been receiving significant attention due to a wide range of potential applications and high absorption in the visible part of the spectrum and high mobility of the charge carriers.1-3 Conductive polymer with polyaromatic backbone including polypyrrole, polythiophene, polyaniline, etc. The received a great deal of attention in the last two decades.4 Amongst the family of conducting polymers polyaniline (PANI) is one of the most promising electrically conducting polymers due to its unique electrical, electrochemical properties, easy polymerization, high environmental stability and low cost of monomer.5
A number of metal and metal oxide particles have been encapsulated into the conductive polymer to form nanocomposites (NCs). The NCs exhibit combination of properties like conductivity, electrochemical, catalytic and optical properties. The NCs are used in applications like electro chromic devices, light-emitting diodes, electromagnetic interference shielding, secondary batteries, electrostatic discharge systems, chemical and bio-chemical sensors.5 The present paper reports the synthesis of polyaniline nanocomposite by the incorporation of n-Al and n-Al2O3 particles in the polyaniline matrix. To achieve this goal, pani, n-Alpani and n-Al2O3pani have been synthesized by in-situ chemical oxidation method. These polymer hybrid nanocomposites have been characterized using various techniques. The structural properties were analyzed by FT-IR and XRD. The interaction of n-Al and n-Al2O3 composite with the polyaniline has been visualized using the Field Emission Scanning Electron Microscope (FESEM). The electrical conductivity measurement was studied using two probe techniques.
Characterization Techniques
FT-IR spectra were recorded on a Bruker Alpha T FT-IR spectrometer. IR spectra of the samples were recorded at room temperature in the mid IR region of 4000-400 cm-1. The XRD pattern was recorded using CuKα radiation (λ=1.54060 A°) with nickel monochromatic in the range of 2Ɵ from 10º to 80º. The FESEM-EDX was recorded using JEOL-Model 6390 machine. Conductivity measurements were performed by a typical Two Probe method with PSM 1735 Frequency Response Analyzer employing the pressed pellet method over the frequency range from 1 KHz to 10 MHz at room temperature. Spectrum of visible light is measured using absorption spectrometer of Stellar Net Inc (model EPP2000). The power of visible light is measured using Newport optical power meter (model 1916-R) and is found to be 600 mW at wavelength of 650 nm (maximum intensity of the light spectrum).
Synthesis of polyaniline
0.1 M of aniline was dissolved in 100 ml of de-ionized water and stirred for 15 min using a magnetic stirrer. 1 M of H2SO4 was added slowly from drop to the aniline monomer solution. 0.1 M of ammonium per sulphate was dissolved in 20 ml of deionized water and slowly added drop by drop for half an hour from a burette vertically to the above prepared solution. After stirring for 5 h, the solution was filtered and the residual was washed with double distilled water, methanol and acetone, and then dried in an oven at 60°C. The final product was grounded into a fine powder.
Preparation of Al and Al2O3 doped with Pani nanocomposites: To synthesize n-Al pani composite, 0.1M aniline monomer and 1M H2SO4 were stirred with double distilled water, and the required quantity (40%) of nano aluminium (Al) powder was added. The oxidant APS was added drop-wise to the aniline-acid-aluminium (Al) mixture with constant stirring. The reaction was conducted at room temperature. When the sample was reacted in the mixed solution of aniline-NH4S2O8–H2SO4, the color of the sample changed to light blue, revealing formation of PANI through an oxidation reaction. The stirring was continued for 5h to ensure complete polymerization. A dark green n-Alpani nanocomposite was thus formed, followed by a color change to dark blue. The composite obtained was filtered and washed with distilled water and methanol to remove excess acid. The product was dried in an oven at 60°C for 12h. The same process was continued with 40% weight of nano Al2O3 with respect to aniline monomer. The dried n-Alpani and n-Al2O3pani composite was fine-ground using a mortar.
FT- IR Analysis
The FT-IR spectroscopy study of pure Pani, n-Al2O3pani and n-Alpani nanocomposites are shown in Figure 1 (A-C). From Figure 1A, the very strong characteristic peak at 3741cm-1 is assigned to the N-H stretching vibration of amino group of polyaniline.6 The absorption band at 2361 cm-1 are correspond to n(N-H)+ unsaturated amine. The very strong peak at 1504 cm-1 is associated with N-B-N stretching vibrations (where B refers to benzenoid ring). The peaks observed in the present work well matches with Pani5-10 Figure 1B & Figure 1C shows all the characteristics peak of Pani with some slight spectral intensity changes. The strong peaks at 2926cm-1 are assigned to asymmetric and symmetric stretching vibration mode of methyl groups.7 The absorption band at 1296cm-1 was assigned to C-N stretching of secondary aromatic amine. The characteristic absorption bands at 1151cm-1 are corresponds to (C=N) stretching vibration.8 The characteristic peak at 1114 cm-1 are due to in-plane bending vibration of C-H mode.9-11 A broad and smooth absorption band in the wave number range from about 400-900cm-1 reveals the formation of Al-O vibrations. The polymerization was confirmed by FTIR spectroscopy. Two additional bands appeared at 615 cm-1 and 593 cm-1 were assigned to Al-O stretching vibrations. This confirms the interaction of n-Al and n-Al2O3 nano particles in the conducting polymer matrix.12-14
XRD Analysis
The X-Ray diffraction patterns of the Pani, n-Al2O3 pani and n-Alpani nanocomposites are shown in Figure 2 (A-C) respectively. XRD studies showed that Pani is amorphous in nature which shown in the Figure 2(A). The broad diffraction peak at 2θ = 24º is characteristic peak for Pani.15
XRD pattern of n-Al2O3 pani and n-Alpani are shown in the Figure 2B & Figure 2C. After doping the samples showed crystalline nature which was confirmed by the peaks at about 2θ = 37.81º, 48.28º, 64.32º and 70.81º for n-Alpani and 2θ = 36.32º, 45.12º and 66.83º for n-Al2O3 pani. These peaks were matched with JCPDS data of Aluminium file no. 04-0787 and Aluminium oxide JCPDS card no. 79-1557. The planes corresponding to n-Alpani is (111), (220), (200), (311) and for n-Al2O3 pani corresponds to (440), (400) and (311). The XRD patterns of n-Al2O3 pani and n-Alpani nanocomposites are compared in Figure 2A. The peak shows sharp and well-defined, indicating the crystallinity of the synthesized materials. The average crystallite sizes have been estimated to be around n-Alpani and n-Al2O3 pani is 31 and 38nm respectively.
FE-SEM Analysis
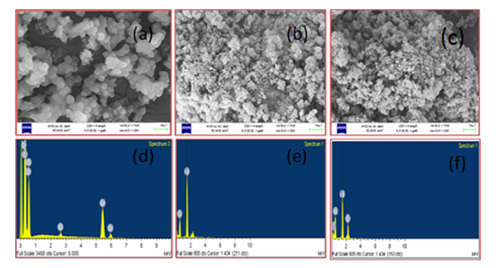
FESEM was performed in order to investigate surface morphology of the polymers and the nanocomposites. EDAX was done to reveal the chemical composition of the samples. Figure 3 (A-F) shows the SEM and EDAX images of Pani, n- Al2O3Pani and n-Alpani with weight percentages are shown in the Tables 1-3. The FESEM image of Pani is spherical and aggregated globules. In the nano composites some of the Aluminium particles seemed to be embedded in the polymer matrix and started coalescing (a tendency to coalesce and form agglomerates) due to the surface absorption property of PANI. The change in morphology can be explained by the absorption and intercalation of PANI on the surface of Al2O3.
Element |
Weight% |
Atomic% |
C K |
55.27 |
69.41 |
O K |
26.87 |
25.33 |
Cl K |
0.59 |
0.25 |
Cr K |
17.27 |
5.01 |
Total |
100 |
100 |
Table 1 Elemental concentration for Pani.
Element |
Weight% |
Atomic% |
Al K |
52.93 |
40 |
O K |
47.07 |
60 |
Total |
100 |
10 |
Table 2 Elemental concentration for n-AlPani.
Element |
Weight% |
Atomic% |
C K |
17.6 |
23.87 |
Al K |
18.8 |
11.35 |
O K |
63.6 |
64.77 |
Total |
100 |
100 |
Table 3 Elemental concentration for n- n-Al2O3 Pani.
The aniline monomer is likely to be absorbed the surface of Al2O3 through electrostatic attraction and by the formation of weak charge-transfer complexes between aniline monomer and the structure of Al2O3.16 The EDX result shows that n-Al and n-Al2O3 was present in the nanocomposite and the weight percentages are shown in Tables (1-3).
C electrical conductivity studies
The A. C Conductivity measurements have been performed by a typical two probe technique. The A. C electrical conductivities of Pani, n-Al2O3 Pani and n-Alpani are shown in Figure 4. The A.C electrical conductivity of Pani, n-Al2O3 pani and n-Alpani were calculated and found to be 8.2 x10-9 S/cm, 1.77x 10-8 S/cm and 2.15 x 10-6 S/cm respectively. when compared to pure polyaniline there is an increase of one order of conductivity in the metal oxide composite and three order increase of conductivity in the nano metal composite. The result shows that nano metal composite posses better electrical conductivity than pure Pani. This enhanced conductivity of n-Al Pani is due to incorporation of metal particles into the polymer matrix. After doping, the increase in the A.C electrical conductivity of n-Al Pani may be due to the even distribution of nano particles and increase in crystallite density in unit space which is evidence from the XRD result. The combination of amorphous and crystalline structure in the composite material may also be the reason for improved conductivity.17
Pure polyaniline, n-Al2O3 Pani and n-Al Pani were synthesized by adopting a facile chemical oxidation polymerization method. The structure of the pure pani and its composites has been confirmed by FT-IR study. The average crystalline sizes have been estimated to be around n-Al pani and n-Al2O3 pani is 31 and 38nm. The FESEM morphology showed that the Pani and n-Al pani and n-Al2O3 pani has the morphological modification due to doping and EDX study reveals that the aluminium and aluminium oxide nanocomposites is evenly distributed through the polymer matrix. The increased conductivity was attributed to the formation of a better charge transport network in the relatively insulating PANI matrix. The improvement in the electrical conductivities of these composites is expected to enhance the potential application of the polymer. The results show enhancement in photo degradation of dyes is due to Al polyaniline crystallite size increase and decrease in particle size, which leads to higher surface and adsorptive nature.
None.
The author declares no conflict of interest.

©2017 Senthilkumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.