MOJ
eISSN: 2574-9773


Research Article Volume 2 Issue 1
1Department of Chemical and Materials Engineering, Chang Gung University, Taiwan
2R&D Center for Membrane Technology, Department of Chemical Engineering, Chung Yuan University, Taiwan
3Department of Radiation Oncology, Chang Gung Memorial Hospital, Taiwan
4Department of Safety, Health and Environmental Engineering, Ming-Chi University of Technology, Taiwan
Correspondence: Shingjiang Jessie Lue, Department of Chemical and Materials Engineering, Chang Gung University, 259 Wen-Hua First Road, Guishan District, Yaoyuan City, Taiwan 333, Tel 88632118800, Fax 88632118700
Received: October 27, 2017 | Published: January 3, 2018
Citation: Hung LY, Hu CC, Hung W, et al. Probing free volume property of polyamide thin film composites with different cross-linking densities. MOJ Poly Sci. 2018;2(1):1-4. DOI: 10.15406/mojps.2018.02.00037
Two commercial polyamide thin film composites (labelled AG and AK) with different polyamide (PA) cross-linking densities were examined for morphology, chemical structure, Doppler broadening positron annihilation spectroscopy, and salt desalination performance. The highly cross-linked AG sample resulted in higher salt rejection but slightly lower water permeance. Meanwhile the highly cross-linked sample showed smaller S parameters than the loosely cross-linked one. The highly cross-linked PA structure resulted in a higher salt rejection and lower water permeation rate. The free volume behavior resulting from various cross-linking densities is captured using the Doppler broadening annihilation spectroscopy.
Keywords: polyamide, cross-linking, thin-film composite, reverse-osmosis membranes, salt rejection, desalination
RO, reverse osmosis; PA, polyamide; PSf, polysulfone; DBES, doppler broadening energy spectroscopy; FTIR, fourier transform infrared spectroscopy; ATR, attenuated total reflective
Reverse osmosis (RO) membranes are commonly used for sea water desalination to produce fresh water. These membranes are usually prepared using interfacial polymerization. Hydrophobic diamine and water-soluble chloride salt precursor are reacted to form a thin polyamide (PA) separation layer on a porous support.1 The PA cross-linking density has a governing effect on the desalination performance of the resulting products.
These delicate PA top layers are less than one micrometer thick, and need to be characterized in order to tailor the material synthesis process. Doppler broadening positron annihilation spectroscopy has been proven to be a useful tool for probing the free volume property along the trans-membrane direction.2 By varying the incident beam energy into specimen, the depth profile of the free volume trait can be obtained.
Two commercial PA thin film composites are tested in this work, and their chemical structure, salt desalination efficiency, water permeance, and the associated Doppler broadening positron annihilation spectroscopy are evaluated. The desalination efficiency is discussed from the chemical structure view point, and supported by the Doppler broadening positron annihilation spectroscopy data.
Material characterization
Two commercial RO membranes (AG and AK models) were purchased from GE Osmosis, Trevose, PA, USA. These membranes have a three-layer structure: polyamide top layer, intermediate layer of polysulfone (PSf), and non-woven support. The membrane morphology was investigated using field-emission scanning electron microscope (S-4800, Hitachi Ltd., Tokyo, Japan). Membrane samples were analyzed for chemical structure on attenuated total reflective Fourier transform infrared spectroscopy (ATR-FTIR, Perkin Elmer Spectrum One, Waltham, MA, USA) and x-ray photoelectron spectroscopy (XPS, Perkin Elmer Phi 1600 ESCA System, Waltham, MA, USA). Doppler broadening energy spectroscopy (DBES) was used to analyze the free volume properties of the PA thin films.2,3
Desalination efficiency
An RO system used in this study consisted of a feed tank, a pump (2SF35SEEL, Cat Pumps, Minneapolis, MN, USA, controlled by a frequency converter), a flat-sheet membrane module, and a permeate tank. A water bath and a heat exchanger were used to control the fluid temperature. The studied RO membrane had a 0.014 m2 of effective area in a flat sheet configuration. A NaCl solution (2 L) was fed into the RO module in a recycle mode at a flow rate of 7.5 L/min. The experimental set-up is detailed in.4 The salt concentrations in the samples were determined using an ion analyzer (IA-300, DKK-TOA Corp., Tokyo, Japan). The water and salt permeance were recorded from the mass flow rates, normalized to the membrane area and applied pressure.4
Morphologies of thin-film composites
The membrane cross-sections were examined using FESEM, as shown in Figure 1. Three layers were visible but the top PA layer was very thin. The total composite thickness was 154±10 mm for AG and 178±1 mm for AK. The non-woven layer was about 93 mm thick. The intermediate layer (made of PSf) accounted for 65±8 mm for AG and 67±5 mm for AK. This intermediate layer is micro-porous (Figure 2). The PA top layer was only a few hundred nanometers thick, with ripple morphology on the surface (Figure 2).

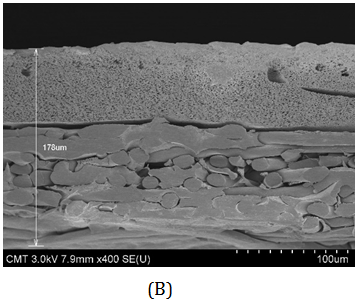
Figure 1 Scanning electron micrographs of cross-sectional views of AG (left) and AK (right) thin film composites. The composites consist of PA top layer, PSf intermediate layer, and non-woven substrate.
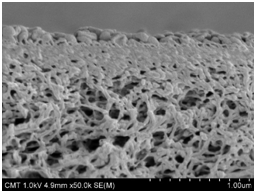

Figure 2 Scanning electron micrographs of cross-sectional views of PA/PSf portions on AG (left) and AK (right) composites.
Chemical structure
The ATR-FTIR spectra of the RO membranes’ PA layers are shown in Figure 3A. Typical amide I and amide II were visible, along with an aromatic ring structure. Figure 1B shows the enlarged view on 2700-3700 cm-1 range. The AK sample had a stronger OH group vibration peak than AG.
The XPS spectra of these membranes are shown in Figure 4. The carbon, oxygen, and nitrogen contents were extracted and the results are summarized in Table 1. Using the previously proposed equation5,6 to calculate the cross-linking density (X in Figure 5) from the XPS data, one could obtained that AG had a higher cross-linking density than AK (0.675 vs. 0.225). The C1s peak deconvolution results (Figure 6) also revealed that AK had more COOH functional groups, with less degree of cross-linking density than AG.
Membrane |
C (%) |
O (%) |
N (%) |
O/N |
X |
Y |
AG |
78.5 |
11.8 |
9.6 |
1.23 |
0.60-0.75 |
0.25-0.40 |
AK |
73.2 |
16.9 |
9.9 |
1.71 |
0.18-0.27 |
0.73-0.82 |
Table 1 XPS results on RO membrane PA payers
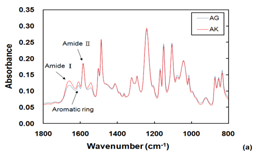
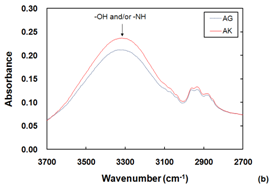
Figure 3 ATR-FTIR spectra of PA layers of RO membranes (A), with enlarged view on 2700-3700 cm-1 range (B).
Doppler broadening positron annihilation spectroscopy
The Doppler broadening positron annihilation spectra of the composites are shown in Figure 7. The AK sample had higher S parameters than AG at the low incident energy range (0-4 keV), which corresponds to the PA top layer.7,8 This implies that the AK sample had higher free volume in the PA constituen.9 In the same energy range, the AK sample showed higher R parameter data than AG, implying that its pore size was larger10 and accounted for the higher free volume in AK.
Salty water desalination performance
The membranes were used for 1.5% NaCl desalination and the results are summarized in Table 2. The AG sample resulted in a lower water flux than AK (2.11 vs. 3.04´10-4 m/s). This is in line with the Doppler broadening positron annihilation spectra: AK had higher free volume and water permeation rate than AG. The highly cross-linked AG sample has a dense structure that rejects salts molecules, rendering slightly higher NaCl rejection (98 vs. 97%).
|
Membrane |
JNaCl (10-3 kg/m2s) |
Jwater (10-4 m3/m2s) b |
Rej. (%)b |
Recovery (%) |
|
AG |
3.88 |
2.11 |
98.16 |
2.3 |
|
AK |
8.15 |
3.04 |
97.32 |
3.3 |
Table 2 Salt permeability, water flux, salt rejection and water recovery through RO membranes
Two commercial polyamide (PA) thin film composites with different PA cross-linking densities were examined for morphology, chemical structure, Doppler broadening positron annihilation spectroscopy, and salt desalination performance. The highly cross-linked AG sample resulted in a higher salt rejection but slightly lower water permeance. Meanwhile, the highly cross-linked sample showed a smaller S parameter than the loosely cross-linked one. The results of the polymer cross-linking structure, salt rejection behavior, and Doppler broadening annihilation spectra are highly correlated to each other.
The financial support from the Ministry of Science and Technology of Taiwan (MOST 103-2221-E-182-064-MY3) is highly acknowledged.
The authors claim no financial interest or any conflict of interest.

©2018 Hung, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.