MOJ
eISSN: 2574-9773


Research Article Volume 1 Issue 3
1Laboratorio de Histologia Integrativa, Universidade Federal do Rio de Janeiro, Brazil
2Laboratorio de Fitoplancton, Instituto de Biologia (IB), Universidade Federal do Rio de Janeiro, Brazil
3Laboratorio de Desenvolvimento Galenico, Universidade Federal do Rio de Janeiro, Brazil
4Laboratorio de Biologia Redox, Universidade Federal do Rio de Janeiro, Brazil
Correspondence: Amanda Santos Franco da Silva Abe, Av Carlos Chagas Filho, 373-Centro de Ciencias da Saude (CCS)-Instituto de Ciencias Biomedicas (ICB) bloco B1-019, Universidade Federal do Rio de Janeiro (UFRJ), Cidade Universitaria-Rio de Janeiro, Brazil, Tel 00-55-21-39386428
Received: June 02, 2017 | Published: June 22, 2017
Citation: Amanda FSFA, Cunha RLD, Salomon PS, et al. Nanoecotoxicological effects of a sunscreen formulation based on Tio2 nanoparticles on microalgae from Guanabara Bay (Rio de Janeiro, Brazil). MOJ Poly Sci. 2017;1(3):99-107. DOI: 10.15406/mojps.2017.01.00014
The rise of nanotechnology has resulted in an increase in the use of nanoproducts, such as sunscreens based on nanoparticles of TiO2 (TiO2-Np), which reach aquatic ecosystems through their manufacture, consumption and disposal. Studies indicate that TiO2-Np have an ecotoxicological potential, inactivating algae from the Anabaena, Microcystis and Melosira genera. Tetraselmis sp., abundant phytoplanktonic organisms of Guanabara Bay (Rio de Janeiro, Brazil), has been exposed to any discarded material released into this environment. In order to evaluate the effects of TiO2-Np on Tetraselmis sp., treatments with different doses of TiO2-Np were performed and their morphology, morphometry, surface charge, chlorophyll a rate and catalase production were analyzed. Under normal culture conditions, doses over 25mg.L-1 caused intense precipitation, agglomeration and disruption of the cells, with remarkable variation of the zeta potential. No significant modification in the amount of chlorophyll a was observed, while the catalase production decreased only by 100mg.L-1 of TiO2-Np. The results showed that TiO2-Np induced several effects in algae, highlighting their cell agglomeration. In conclusion, TiO2-Np provoked harmful effects in Tetraselmis sp., an important phytoplanktonic organism of Guanabara Bay (Rio de Janeiro, Brazil), and may disturb the dynamics of this ecosystem.
Keywords: TiO2 nanoparticles, tetraselmis sp, guanabara bay, environmental nanotoxicology
Among the most common manufactured nanoproducts, sunscreens based on titanium dioxide nanoparticles (TiO2-Np) stand out. They have been available to consumers since 2009, with ca. 7,500 tons sold per year by 2011.1 The increasing production of cosmetics based on TiO2-Np is explained by the fact that they generate formulations that are safer and more stable, providing high UV protection2 while reducing undesirable opacity. TiO2-Np sunscreens are more transparent and commercially attractive than conventional sunscreens.3 The properties that make Nps so unique may also be responsible for their toxic potential.4 Although there is currently great exposure to these nanomaterials, in vitro studies have revealed adverse effects on human cells and tissues5 increase of the production of reactive oxygen species (ROS) and damage in A431 human cells6 and cytotoxicity in human skin fibroblasts.7 Most studies concerning these nanoproducts have focused on analyzing their activities exclusively on human health. However, questions about the environmental consequences of their large-scale production and quotidian use remain unanswered. For example, information about the degradation or accumulation of nanomaterials in aquatic environments is still scarce.
The exact amount of TiO2-Np dispersed in aquatic environments is difficult to define since it varies depending on sewage treatment systems, water pollution, consumption patterns and disposal regimes of Np-based products in different countries. Considering that almost 100% of wastewater in Switzerland enters sewage treatment plants, the predicted environmental concentration values for TiO2-Np in water are 0.7-16 µg/L.8 It has been estimated that up to 10% of the Np pass through wastewater treatment and are discharged into Swiss waters.9 At one wastewater treatment plants in Arizona (USA), raw sewage contained 100 to nearly 3000 µg TiO2-Np/L.10
In Brazil, there are no records of the concentration of TiO2-Np in sewage or in aquatic environments. However, it is well known that Brazilian sewage treatment is not as efficient and does not reach as many areas11 as in Switzerland or the USA. In addition, high temperatures, a high incidence of solar radiation throughout the year and the extensive Brazilian coast favor the consumption of sunscreens based on TiO2-Np and the consequent release of these Np into the water. In this context, the toxic potential of Np to aquatic organisms should not be overlooked. The acute toxicity of TiO2-Np to the microcrustacean Daphnia magna is dose-dependent12 and TiO2-Np induced severe oxidative effects in the liver of zebrafish at 50 mg/L.13 Although the potential for TiO2 impacts on natural receiving systems appears to be relatively low, algae and invertebrates seem to be more susceptible to TiO2 toxicity.14 TiO2-Np were more toxic to the algae Pseudokirchneriella subcapitata then the non-nano form of TiO2, since the TiO2-Np "confine" these algae.4 Moreover, when associated with UV light TiO2-Np inactivated algae of the genera Anabaena, Microcystis and Melosira.15
In Brazil a law project (number 1,370-A/2011)16 that prohibited the use of TiO2 in food products and cosmetics was presented. Although the above-mentioned studies demonstrated the hazardous effects of TiO2, the project was rejected. According to members of the Congress, the project was refused "since there is neither concrete evidence of environmental impacts of TiO2 in cosmetics nor scientific data about the toxicity of TiO2-Np in aquatic organisms". Consequently, further research into the effects of products that contain TiO2-Np on organisms of aquatic ecosystems is necessary. Phytoplankton are the dominant primary producers in marine ecosystems,17 where they are the base of oceanic food webs and a dominant component of the global carbon cycle as well as other biogeochemical cycles.18 The green microalgae Tetraselmis sp., as a planktonic population of Guanabara Bay (Rio de Janeiro, Brazil), is constantly exposed to any material introduced into this aquatic environment. Given that these algae are important in such environments, Tetraselmis sp. is suitable organisms for toxicity tests with nanoproducts. In this context, the present study aimed to evaluate the effects of different doses of a sunscreen formulation based on TiO2-Np on the algae Tetraselmis sp., improving the knowledge about the toxicity of TiO2-Np on aquatic microorganisms.
Cell isolation and cell culture
Non-axenic cultures of Tetraselmis sp. microalgae (Figure 1) were obtained from Guanabara Bay (22050'40''S 43012'40''W, Rio de Janeiro, Brazil; (Figure 2) in May, 2013 and December, 2015. Cells were cultivated in marine Guillard f/2 media (35%0 salinity) made with filtered (0.22 mm) natural seawater, which was autoclaved prior to inoculation. The algae were maintained in a germinating camera (BOD incubator with photoperiod-Electrolux Cienlab, BRA) with 100 mE/m2/s irradiance, at 22°C. The photoperiod was 16h light: 8h dark. Cells (an initial number of 2.6 x 104/ sample) in exponential growth were exposed to various concentrations of TiO2-Np under cultivation conditions. The assays were performed in triplicate.
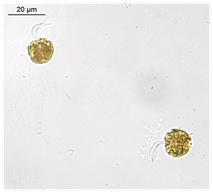
Figure 1 Microalgae Tetraselmis sp., an important representative of the phytoplankton from Guanabara Bay (Rio de Janeiro - Brazil), appropriate for nanoecotoxicological studies. Cells are fixed with lugol (1%). Bars = 20 (A) and 100 (B) μm.
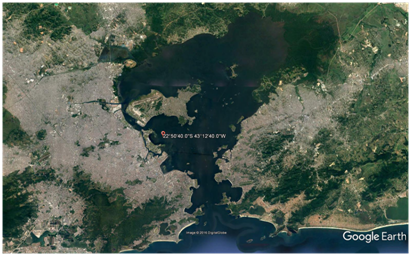
Figure 2 Guanabara Bay, an important aquatic ecosystem of Rio de Janeiro (Brazil) is exposed to all kinds of discarded or released materials. Source: Google Earth.
Nanoparticle characterization
The mean diameter of the TiO2-Np was evaluated by dynamic light scattering (DLS-Zetasizer Ver. 6.20, Malvern, GBR). Their morphology was assessed through transmission electron microscopy (TEM - Morgagni 268 D, FEI, USA). The zeta potential (average surface charge) of Np diluted in distilled water or in F2 medium was analyzed in a Zetasizer Nano ZS (Malvern, U.K., using the Malvern Software v. 7.03). Electrophoretic mobility values were calculated from the average of readings performed in triplicate for each sample.
Experimental procedures
All methods described were performed in triplicate and statistically analyzed in the software GraphPad Prism (version 4), using non-parametric one-way ANOVA tests with Tukey post tests. The initial cell number was 2.3 x 104 cells/well and the treatments with TiO2-Np (ranging from 1 to 100 mg.L-1 diluted in F/2 medium) lasted 30 minutes (acute effect) or 96 hours (chronic effect). Preliminarily analysis focused on the effects of Np in the absence or presence of light, since aqueous Np suspensions are opaque and may inhibit algal growth by absorption of light.4 The tests were performed in culture conditions, under the germinating chamber lamp, in order to simulate the conditions of natural ecosystems. Light irradiation treatment was at two levels, exposed and blocked. Both exposed or blocked cells remained inside the germinating chamber, the blocked cells being covered during the experiment.
Cell cluster morphometry and determination of the RGB intensity
Cells were exposed to several different doses of a sunscreen formulation based on TiO2-Np (Eusolex T-aqua®, 30% of TiO2-Np Merck, USA) in 24-well plates (TPP, SUI). After treatments, the samples were photographed at a determined distance (5.5 cm), using a digital still camera (Sony DSC-W350D, USA). The registered cell clusters were outlined and analyzed in the software Image J, which provides information on the red, green and blue (RGB) intensities of each image as well as the area of the image (in pixels). The greater the magnitude of RGB, the greater the intensity of the colors red, green and blue, as well as the density of the cell flake.
Cell morphology
After treatments, the samples were fixed with 1% lugol and analyzed using inverted optical microscopy (Olympus IX70, software Olympus cellD 3.2) in order to obtain the micrographs (XC50 camera) from the central region (central region) and of the edges (peripheral region) of the wells.
Turbidity decay
The turbidity decay kinetics, measured using spectrophotometry (v-630 Jasco, JPN) and assessed with Spectra Manager software, may be applied as an indirect measurement of the cell density decrease. The test was performed for 1 h with readings at 640 nm, every 10 minutes, using F/2 medium as a blank. The rate of absorbance decay is calculated through the formula: (absorbance of time 0 - absorbance of time x) *100.
Non-flocculated cell counting
Non-flocculated cells were fixed with lugol (1%) and counted in a Palmer-Maloney chamber using a light microscope (DM 750 Leica, DE).
Concentration of active chlorophyll a (Chla) through spectrofluorometry
Since the concentration of chlorophyll is correlated to the concentration of phytoplankton, it is used to estimate the primary productivity and biomass in the ocean. In addition to chlorophyll a, marine samples also present chlorophyll b and c, along with their degradation products, pheophytin a (phe a), pheophytin b and pheoporphyrin c [19]. After treatment, the samples were filtered (filter Whatman GF/F diameter = 47 mm, pore = 22 µm) and the filters were incubated in glass tubes with 5.4 mL of 100% acetone (Merck, USA) for 16 h at 40C, for pigment extraction. The extracted pigments were diluted (1:10 at acetone 90%), centrifuged and analyzed in a spectrofluorometer (Varian Cary Eclipse, using the calculation software MATLAB-Agilent Technologies, USA) using 90% acetone as a blank. Concentrations of Chla were assessed using a modified version of Neveux & Lantoine’s20 method, described by Tenório et al.21 Data acquisition was performed by recording the fluorescence emission spectra for each of 31 excitation wavelengths (3-nm increments from 390 to 480 nm). Pigment concentrations were estimated from the resulting data points. The least-squares approximation technique was constrained to discard negative solutions. Active Chla was represented by the chlorophyll a/pheophytin a ratio, expressed as a percentage, as described by Neveux & De Billy et al.22
Zeta potential of the cells
In order to evaluate the zeta potential of the cells (average surface charge), samples were diluted after treatment (1:20 in F/2 medium) and analyzed in a Zetasizer Nano ZS instrument (Malvern, U.K., using the Malvern Software v. 7.03) at 250C.
Catalase (CAT) activity
After treatment, the samples were sonicated (sonicator UltraCleaner 1600A, Unique, BR) at potency 4, three times for 10 seconds, with a two-second interval between the sonications. They were then incubated in a microplate with H2O2 (hydrogen peroxide) in phosphate buffer (PBS) (0.16%) at a proportion of 3 µL of sample / 97 µL of the peroxide solution. CAT activity was evaluated spectrophotometrically (Flex 3 station, using the software Softmax 5) by measuring the consumption of H2O2 at 240 nm, 0, 30 and 60 seconds after incubation with H2O2. The calculation of the CAT activity was made using the extinction coefficient 0.0436 mM-1 cm-1 (method adapted from.23) The results were expressed as U/mg protein.
The measurement of particle size is usually used to define materials as nano-sized. This classification seems straightforward but is subject to several difficulties. The dynamic light scattering (DLS) analysis showed that the TiO2-Np exhibited an average diameter of 220.9 nm (Figure 3A) with a polydispersity index of 0.160, demonstrating a homogeneous size distribution. However, in transmission electron microscopy (TEM) individual Np exhibited diameter of around 100 nm, although they tended to agglomerate forming clusters. Thus, their clustered arrangement may mask the real diameter of Np. The images also revealed that most Np had elliptical morphology with a smooth surface (Figure 3B). These results corroborate the observations of Jacobs and coworkers 24 who noticed that TiO2-Np tend to agglomerate and aggregate into larger structures. Thus, to refer to TiO2-Np as monodisperse may be a misnomer under common environmental conditions.25
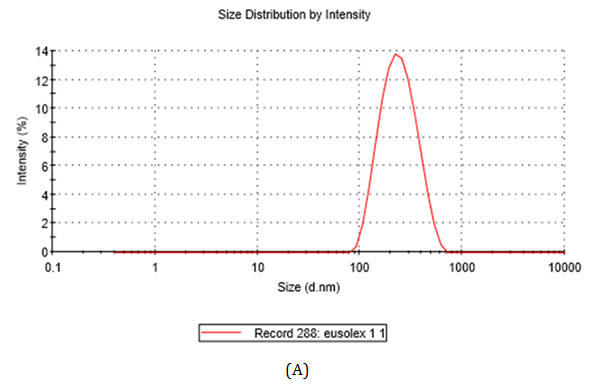
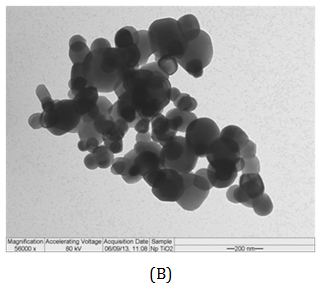
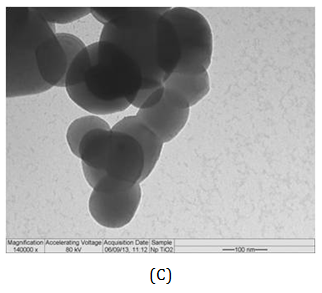
Figure 3 Size distribution (A) of TiO2-Np (B, C). Their average diameter, obtained through the DLS method, was 220.9 nm (A). Electron micrographs revealed Np of different sizes (B, C). Bars = 200 (B) and 100 nm (C).
Since aqueous suspensions of Np are opaque, and the inhibition of the algae growth could in part be due to the absorption of light,4 the effect of TiO2-Np on Tetraselmis sp. in the presence or absence of light irradiation from the germinating chamber was evaluated (Figures 4, 6 and 7). The results showed that the TiO2-Np formulation induced cell agglomeration with the consequent formation of macroscopic clusters (Figure 4), and significant clusters were formed at doses above 10 mg.L-1 in the dark and above 25 mg.L-1 under light irradiation (Figure 5). Increasing doses enhanced the cluster area in a dose-dependent manner under both conditions suggesting that either in the dark or under irradiation the algae precipitate and agglomerate, losing their ability to swim due to the flocculating action of TiO2-Np. At the highest dose (100 mg.L1), the cells flocculated around 14.9 and 18.3 times more than untreated cells, in the absence or presence of light, respectively. This effect was also described in the algae P. subcapitata that carried 2.3 times their weight in Np adhering to their surface.26 Moreover, TiO2-Np may reduce the availability of light to algae entrapped clusters, inhibiting their growth 4. The algae Chlamydomonas reinhardtii27 and Dunaliella tertiolecta18 also showed TiO2-Np aggregates on their cell surface. If this effect also occurs in Tetraselmis sp., we suggest that the occurrence of Np aggregates on algal surfaces may be one of the reasons for the cell precipitation observed in this study. In light microscopy, the wells revealed two distinct areas: the central and the peripheral regions. The central region represented the area with clumped cells in contrast to the peripheral region, in which only non-flocculated cells (free cells) or a small number of tiny cell clusters among cell fragments could be visualized. After 30 minutes, 5 mg.L-1 TiO2-Np caused a reduction of the cell number in comparison with the control. Treatments with doses higher than 25 mg.L-1 TiO2-Np provoked an even more intense decline in the number of cells, as well as an increase of the amount of cell fragments, indicating cell rupture associated with extravasation of cytoplasmic contents and consequent cell death (Figure 6). Zhu et al.12 reported that TiO2-Np also exhibited a gradual increase in toxicity to the microcrustacean D. magna, inducing 100% immobilization and 90% mortality, at 500 mg.L-1 and 20% immobilization and 0% mortality at 10 mg.L-1.
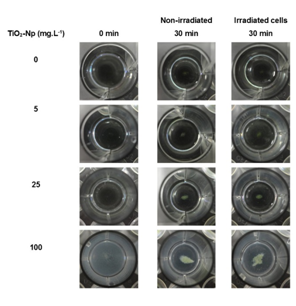
Figure 4 Macroscopic view of the cell clusters after 30 minutes of interaction of Tetraselmis sp. with the sunscreen formulation based on TiO2-Np. The cell-Np interaction resulted in a dose-dependent increase in cell clusters in the presence or absence of light irradiation.
Hund-Rinke et al. 28 explained that part of the algal inhibition by TiO2-Np is probably not caused by the reduced light intensity, but is due to the toxicity of the particles. The sensitivity of other algae to TiO2-Np has been previously described29 for example, P. subcapitata is more sensitive to nanosized TiO2 than C. reinhardtii, followed by D. subspicatus. Tetraselmis sp. is a native alga of Guanabara Bay, an important Brazilian hydric ecosystem, and the present work is the first report concerning its susceptibility to TiO2-Np. Cell precipitation provoked by TiO2-Np also resulted in increasing cell density in the clusters (represented by RGB intensity) at doses higher than 1 mg/L (Figure 7). This result indicates that microalgae tend to adhere to each other even after exposure to lower doses of TiO2-Np. Nevertheless, they do not form significant cell clusters at low doses. The kinetics of turbidity decay was assessed as an indirect representation of the progressive decrease in the amount of cells in the water column. Using this method, which analyzes absorbance decay, a gradual reduction in the turbidity of the samples was observed, indicating microalgae precipitation. In addition, the turbidity decay was significant at all concentrations, as observed in RGB analysis (Figure 7), showing that the cells tend to precipitate even at low doses of TiO2-Np (Figure 8). Nevertheless, the percentage of turbidity decay increased with the TiO2-Np dose and with the time of cell-Np interaction. After 10 minutes of treatment with 100 mg.L-1 TiO2-Np the turbidity decreased 2.84% in comparison with the control, increasing to 28.41% after 60 minutes of interaction (Table 1). The aggregation behavior of TiO2-Np depends strongly on the ionic strength of the medium because cationic and anionic species affect the stability of TiO2 suspensions.30 Guilliard f/2 medium, in which the microalgae were grown, presents metals that dissociate into ions that may intensify the flocculating potential of TiO2-Np. Furthermore, TiO2-Np can adsorb zinc and phosphorus from the growth medium and thus limit the availability of these nutrients to algae,31 impairing their survival.
Time (min)/TiO2-Np (mg.L-1) |
0 |
1 |
5 |
10 |
25 |
50 |
100 |
10 |
0.26 |
0.81 |
1.53 |
1.22 |
1.84 |
1.23 |
2.84 |
20 |
1.72 |
2.92 |
1.38 |
2.68 |
4.29 |
4.73 |
5.44 |
30 |
1.67 |
3.15 |
3.14 |
2.71 |
5.3 |
6.9 |
9.62 |
40 |
2.5 |
3.64 |
4.26 |
3.39 |
4.94 |
7.74 |
14.09 |
50 |
3.04 |
4.54 |
4.94 |
6.08 |
7.04 |
8.8 |
18.25 |
60 |
4.31 |
5.59 |
5.51 |
6.24 |
6.75 |
12.07 |
28.41 |
Table 1 Rate of turbidity decay (%) compared to control cells during 60 minutes of interaction between algae and Np
Cells outside the clusters (free cells) were also measured after 30 minutes (acute effect) and 96 hours (chronic effect) of treatment under normal culture conditions, in order to simulate the conditions in which the algae live in the natural environment. The results indicated that the amount of free cells was progressively reduced in a dose-dependent manner at concentrations greater than 25 mg.L-1. However, after 96 hours of treatment, this effect was significant at doses from 10 mg.L-1 TiO2-Np (Figure 9). At doses greater than 25 mg.L-1 TiO2-Np, there was a reduction in the number of free cells as well as significant formation of algae clusters that could be visualized, indicating that cells were incorporated into the clusters (Figure 6B).
As well as Tetraselmis sp., P. subcapitata treated with TiO2-Np revealed no variation in growth after 96 h,32 except for those cells treated with 100 mg.L-1 TiO2-Np. Although both algae were susceptible to TiO2-Np displaying chronic effects, P. subcapitata was probably more sensitive than Tetraselmis sp., since P. subcapitata showed a decrease of 25% in the cell number at 1 mg.L-1 of TiO214 compared to Tetraselmis sp. that exhibited the same inhibition at 25 mg.L-1. Usually, TiO2-Np can suppress the growth of pelagic algae, but these impacts are manifested by contrasting effects on species-specific metabolic functions. As the growth and metabolism of algae are essential to the balance of ecosystems and the organization of aquatic food webs, TiO2-Np can cause significant alterations in the ecosystem properties of freshwater habitats.33 Considering the huge importance of a fully functional photosynthetic metabolism in microalgae, the amount of active chlorophyll a (Chla), a crucial pigment in photosynthesis, was investigated. Despite the intense cell flocculation, the amount of active Chla after 30 minutes of cell-Np interaction did not vary significantly, suggesting that their photosynthetic metabolism was not affected (Figure 10). Thus, TiO2-Np affect algal swimming and survival after a short time of interaction but not their capacity to produce photosynthetic pigments such as Chla. As for Tetraselmis sp., C. reinhardtii treated with TiO2-Np demonstrated no significant modifications in their chlorophyll a content, although the authors noted an inhibition in the photosynthetic efficiency (followed by recovery to normal levels) and in cell growth. Other similarities with Tetraselmis sp. were observed in C. reinhardtii, i.e., as the concentration of TiO2-Np increased, the number of damaged cells also increased.27 Most microalgae have negatively charged cell surfaces, caused by the ionization of ionogenic functional groups on the cell walls and also by the adsorption of ions from the culture medium. The species of algae, the ionic strength of the medium, the pH, and other environmental conditions have a great impact on the development of this property. The negative charge of the cell surface is significant for microalgae growth, mainly for preventing the natural aggregation of suspended cells. At neutral pH, microalgae reveal a slightly negative charge because of the presence of proton-active carboxylic, phosphoric, phosphodiester, hydroxyl, and amine functional groups.34
Analysis of the zeta potential of the microalgae (Figure 11) showed that control cells displayed a -8.94 mV mean membrane charge, which remained unchanged after treatment with 1 or 5 mg.L-1 TiO2-Np. Cells treated with 10 or 25 mg.L-1 of TiO2-Np revealed a reduction in the average charge of the cell surface, reaching a minimum value of -13.95 mV, while treatments with 50 or 100 mg.L-1 of TiO2-Np induced a significant increase in the average charge. These differences among the zeta potential values might be due to the rupture of cell membranes with consequent extravasation of cytoplasmic content visualized in light microscopy (Figure 5). These effects became more prominent at doses above 25 mg.L-1. Thus, the dispersed cytoplasmic material modified the average charge, making it more positive. Although the average charge of Tetraselmis sp. treated with TiO2-Np is negative, the cells tend to clump together forming clusters. This may be explained by the destabilization of colloidal systems (such as the Np-TiO2 based sunscreen formulation) as explained by Henderson, Parsons et al.35 They reported that there is electrostatic repulsion between adjacent particles if the colloidal stability of the system is maintained. Disruption of the system stability through water treatment with the addition of cationic chemicals, such as Al3+ or Fe3+, induces neutralization effects. In destabilizing the system, attractive van der Waals forces dominate over repulsive electrostatic forces. Considering that f2 medium, wherein the TiO2-Np formulation was solubilized, contains water and Fe3+, we suggest that the disturbance of the formulation stability might explain the aggregation of the cells. Thus, the presence of intracellular contents in the samples, as evidenced in the micrographs, may explain the increase of the average charge at doses higher than 25 mg.L-1.
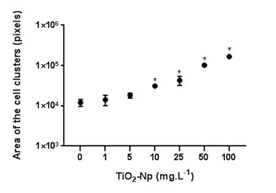
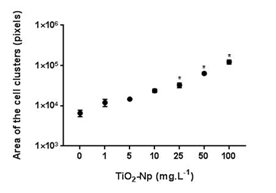
Figure 5 Morphometry of cell clusters after 30 minutes of interaction of Tetraselmis sp. with TiO2-Np in the absence (A) or presence (B) of light irradiation. Significant cell clusters were formed at doses starting from 10 (nonirradiated cells) and 25 (irradiated cells) mg.L-1 TiO2-Np (p>0.05).
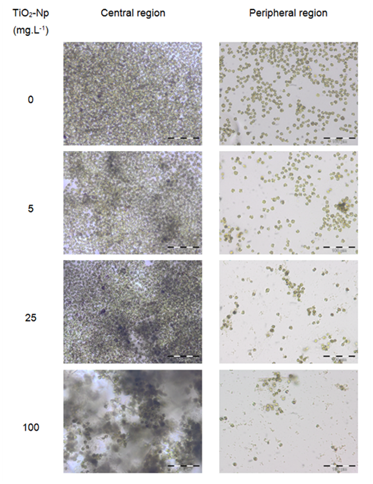
Figure 6 Microscopic analysis of the interaction between the microalgae Tetraselmis sp. and the TiO2-Np sunscreen formulation. The "central region" presents cell clusters with increasing cell density, in proportion to the increase in Np dose. The "peripheral region" shows a dose-dependent reduction in cell number and the formation of cell fragments, indicating cell disruption and death. Bars = 100 μm.
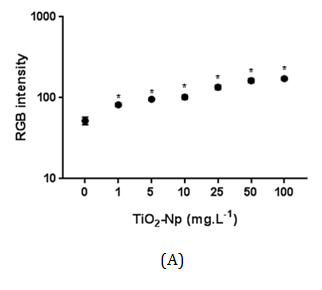
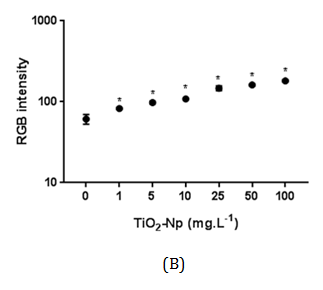
Figure 7 Intensity of RGB of Tetraselmis sp. treated with increasing doses of TiO2-Np in the absence (A) or presence (B) of light irradiation. Significant cell density enhancement is observed at doses above 1 mg.L-1, suggesting that these Np cause an agglomerating effect in microalgae (p>0.05).
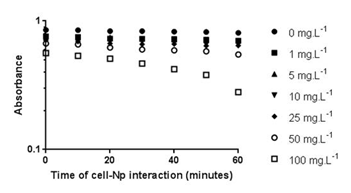
Figure 8 Kinetics of turbidity decay as an indirect representation of cell density diminishing in the water column. Microalgae precipitation is stimulated by increasing doses of Np (p>0.05).
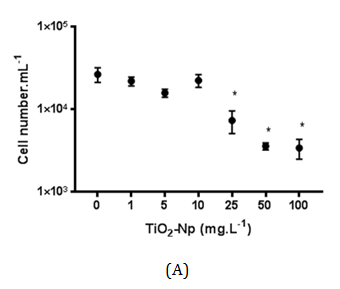
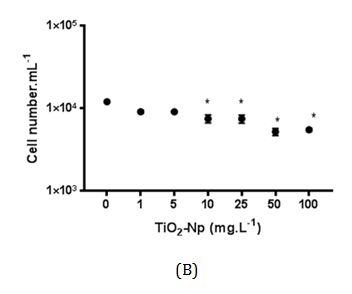
Figure 9 Effect of TiO2-Np on non-flocculated microalgae after 30 minutes (A) and 96 hours (B) of interaction with Np. The acute treatment (30 minutes) showed a significant decrease in the cell number at doses greater than 25 mg.L-1. In the chronic treatment (96 hours), 10 mg.L-1 of TiO2-Np was enough to induce a significant reduction in the cell number (p>0.05).
The average charges of TiO2-Np (100 mg.L-1) diluted in distilled water and in f2 medium were significantly different, suggesting that f2 medium alters the average charge of Np as shown in Table 2. All aerobic organisms contain catalase, a tetrameric, heme-containing enzyme. Because of its wide distribution and capacity to rapidly degrade hydrogen peroxide, it has been proposed that catalase plays an important role in the systems that enable organisms to live in aerobic environments.36 Considering that TiO2-Np induce the generation of reactive oxygen species (ROS),1,13 the ability of microalgae to mitigate oxidative stress was assessed by measuring the production of catalase after 30 minutes (acute effect) and 96 hours (chronic effect) of cell-Np interaction (Figure 12). At 30 minutes, the production of catalase was not significantly altered, indicating no response of the cells to the probable oxidative stress induced by TiO2-Np. Nevertheless, at 96 hours, catalase activity was significantly reduced in cells treated with 100 mg.L-1 of TiO2-Np. It is important to note that control cells at 96 hours showed higher amount of catalase in contrast to those at 30 minutes. This result indicates that the culture conditions may induce the production of catalase even in control cells. The production of ROS by TiO2-Np has usually been attributed to the presence of ultraviolet light (UV).37 Miller and coworkers18 related that under low intensity UV, there is an increase of ROS in seawater with increasing TiO2-Np concentration. However, TiO2-Np promoted the production of ROS in P. subcapitata when exposed to UV, with or without a UV filter38 and in fish cells in the absence of UV.39 In algae, an overall enhancement in the activities of antioxidant enzymes, such as catalase, following exposure to environmental stresses is evident. Nonetheless, a species- and stress-specific reduction in some antioxidant responses has been recorded.36
TiO2-Np (100 mg.L-1) |
Standard deviation |
Zeta potential (mV) |
in f2 medium |
0.22 |
-1.31 |
in distilled water |
3.4 |
-11.6 |
Table 2 Zeta potential values of TiO2 nanoparticles diluted in F2 medium or in distilled water
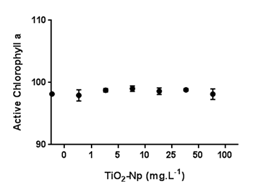
Figure 10 Chlorophyll a fluorometric analyses. Treatments with several doses of TiO2-Np did not significantly alter the photosynthetic metabolism of Tetraselmis sp. after 30 minutes of cell-Np interaction (p>0.05).
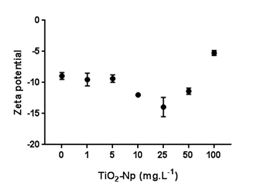
Figure 11 Average microalgal surface charge (zeta potential) of the samples after treatments with different doses of TiO2-Np. Treatments at 10 and 25 mg.L-1 TiO2-Np caused a reduction in the average charge, while higher doses stimulated an increase in charge (p>0.05).
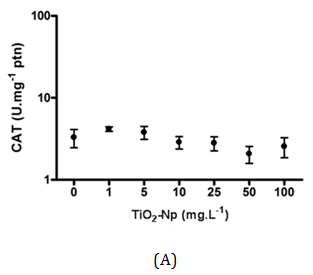
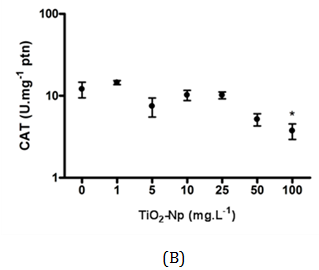
Figure 12 Measurement of catalase activity after interactions between Tetraselmis sp. and TiO2-Np. At 30 minutes, the amount of catalase showed no significant change (A). At 96 hours, cells in contact with Np exhibited higher amount of the enzyme. At 100 mg.L-1 TiO2-Np, the concentration of catalase decreased compared with control cells (B) (p>0.05).
In this work, the cells were irradiated with a regular lamp from the germinating chamber, but not with a UV source. Unexpectedly, the catalase production in acute and chronic treatments was basically the same in all treatments, except in those submitted to 100 mg.L-1 TiO2-Np for 96 h. This does not necessarily mean that there was no production of ROS induced by TiO2-Np. Despite the presence of ROS, it is possible that cells did not respond efficiently to the oxidative stress (through catalase synthesis), although they underwent membrane rupture, an effect often associated with membrane lipid peroxidation and previously reported in zebrafish cells,13 as well as in the algae N. oculata 40.
At present, the exact concentration of Np in Brazilian aquatic environments is unknown. According to the European Union Directive on classification, packaging and labeling of dangerous substances (Council Directive 67/548/EEC), a toxicity in the range of 10-100 mg.L-1 combined with a lack of biodegradability has to be classified as 'harmful to aquatic organisms and may cause long-term adverse effects in the aquatic environment'.28 Our results demonstrate that even low doses such as 1 mg.L-1 of a TiO2-Np sunscreen formulation is enough to induce significant cell sedimentation. This effect is intensified at doses over 10 mg.L-1 (in the dark) or 25 mg.L-1 (under irradiation), and is capable of increasing cell clusters in a dose and time-dependent manner. In natural environments, these effects are extremely deleterious, considering that planktonic algae need to remain on the water surface to capture light and perform photosynthesis. Furthermore, doses greater than 25 mg.L-1 of TiO2-Np induced cell disruption with extravasation of cytoplasmic contents, resulting in alterations in the mean charge (zeta potential) at increased TiO2-Np doses. An acute effect on the amount of chlorophyll a was not observed, nor was antioxidant metabolism (catalase production) significantly activated, except at 100 mg.L-1 TiO2-Np under chronic treatment (96 h). Nevertheless, the ROS amount was not evaluated in this study. Further investigations should be performed to assess chronic changes in photosynthetic metabolism and the production of ROS in Tetraselmis sp. exposed to TiO2-Np. We have demonstrated harmful effects induced by a formulation based on TiO2-Np on an organism with ecological importance in Guanabara Bay (Rio de Janeiro, Brazil). Thus, the indiscriminate use of products containing TiO2-Np may disturb the dynamics of the microbiota in this ecosystem. This work should thus sound a warning about the nanotoxicological effects of indiscriminate use of nanoproducts based on TiO2-Np, and contribute to the future development of strategies for reducing environmental risks.
Denize Ferreira da Silva, Dr. Cristal dos Santos Cerqueira Pinto, Dr. Álvaro Augusto da Costa Piglet, Funding agencies CNPq and FAPERJ
The author declares no conflict of interest.

©2017 Amanda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.