MOJ
eISSN: 2574-9773


Research Article Volume 2 Issue 1
1Membrane Science and Separation Technology Division, Council of Scientific & Industrial Research-Central Salt and Marine Chemicals Research Institute, India
2Academy of Scientific and Innovative Research, Council of Scientific & Industrial Research-Central Salt and Marine Chemicals Research Institute India
Correspondence: A Bhattacharya and Saroj Sharma, 1Membrane Science and Separation Technology Division, Council of Scientific & Industrial Research, Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Bhavnagar, Gujarat, India, Tel 91-278-2567760-7610
Received: January 01, 2018 | Published: January 31, 2018
Citation: Mehta R, Bharda PS, Sharma S, et al. Mitigation of arsenic from water through tailor-made thin film composite (TFC) membrane. MOJ Poly Sci. 2018;2(1):29-32. DOI: 10.15406/mojps.2018.02.00042
Drinking arsenic contaminated water is a major threat to mankind. In the past few decades membrane technology has been emerging as potential technique to counter the water contamination problem. In this regard ‘Thin film polyamide composite’ membrane shows remarkable behaviour. Our laboratory made thin film composite membrane shows good potential in removing arsenic (As(V) 92.72%) from water. It also shows that As(V) removal is much more (~14%) compared to As(III) for this membrane.
Keywords: thin film polyamide composite membrane, polysulfone, arsenic, water
TFC, thin film composite; MPD, 1,3-phenylene diamine; TMC, 1,3,5 trimesoyl-chloride
The inferno of ‘water contamination’ is a major threat to mankind. Arsenic is a toxic and carcinogenic metalloid that is infiltrated into aqueous system through natural as well as anthropogenic sources. Arsenic contamination is by far the biggest ‘mass poisoning’ in the world. It can exist in different form viz. As (III) and As (V) in natural water.1 Human exposure to arsenic can take place through ingestion, inhalation or skin adsorption. High dose of arsenic can cause acute toxic effects including gastrointestinal symptoms (poor appetite, vomiting, etc.), disturbance of cardiovascular and nervous systems functions (e.g. muscle cramps, heart complains) or death.2–4 EPA, US, 2006 has fixed MCL of arsenic in drinking water as 0.01mg/L.5
There are different techniques (like oxidation, ion exchange) to mitigate and remove arsenic from water.6 Among available technologies applicable for water treatment, membrane filtration has been identified as a promising technology to remove contamination from water. It is preferred because of their attractive features viz. low operating costs, low environmental impact and no requirement of chemical addition. Membranes from different polymeric materials are used for different applications.7–9
Research to address this problem has worked towards developing membranes by coating diamine and trimesoyl chloride over the asymmetric polysulfone substrate. It is called as ‘thin film polyamide composite’ membrane. The undisclosed composition of commercial membranes drives us to prepare ‘thin film composite membranes’ in our laboratory. Thin film composite membrane has been used in desalination as well as removing organics from water. We report here the potentiality of tailor-made thin film composite membrane in terms of Arsenic removal.
Non-woven Polyester fabric (PGI, France), Polysulfone (Udel P-3500; Solvay advanced Polymers, USA), 1,3-phenylene diamine (Sigma, USA) and 1,3,5 trimesoyl chloride (Sigma, USA) were used to prepare thin film polyamide composite membrane. N,N Dimethyl formamide (Loba, India) and n-hexane (Merck, India) were used as solvents. Potassium Chloride (Rankem, India) was used for zeta potential studies. All of the above chemicals were laboratory reagent grade and used as received. In the experiment deionized water was used.
The salt rejection performance study was used using sodium chloride (Rankem, India) to mark the membrane. Sodium Arsenite (NaAsO2) (As III) and Disodium Arsenate (Na2HAsO4) (As V) (both purchased from Sigma Chemicals, USA) were used for filtration experiments.
Preparation of thin film polyamide composite membrane
Polysulfone 15(%w/w) in N, N-dimethyl formamide was prepared by continuous stirring at 65°C for 6h. The conical flask was capped and properly sealed before using it. Polysulfone membrane was prepared by pouring the solution on nonwoven polyester fabric mounted on prototype casting machine and was dipped into non-solvent water bath. The machine is of two parts viz. casting chamber and water bath. Polysulfone solution was poured on non-woven polyester fabric in the casting chamber at temperature 25°C and relative humidity 30%. The polysulfone thickness was controlled by aligned micrometer in the casting machine. The motor speed was controlled and kept at 2m/min. The polysulfone casted membrane was dipped in water bath to phase out. The proto type casting machine was sketched in Figure 1.
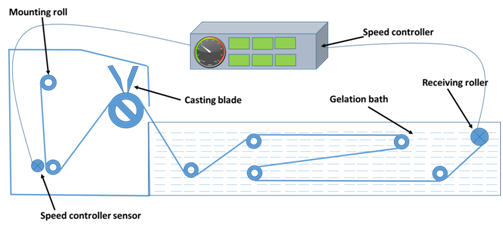
1,3-phenylene diamine solution in water (2%w/v) was poured on prepared Polysulfone membrane. The membrane was dipped for 1min and decanted. After gap of 1min 1,3,5 trimesoyl chloride (0.1%w/v) in hexane again poured on to it. Again the membrane was dipped for 1min and decanted. Interfacial polymerization of 1,3-phenylene diamine and 1,3,5 trimesoyl chloride in water-hexane interface took place. The polyamide formation was cured at 80°C for 2 min to form the crosslinked polyamide. The reaction was depicted in Figure 2.
The separation performance experiments were carried out in laboratory made pressure cell using cross-flow filtration mode. The experimental set up was based on four pressure cells, connected in series where membranes were fixed. The pressure was applied and feed passed parallel to the membrane. The detail of the set up was sketched elsewhere.10 The effective membrane area was 0.00152m2 for this particular cross-flow filtration unit. In all the experiments applied pressure was kept at 1.34MPa. The salt rejection experiments were done with sodium chloride. The salt rejection performance was done by their conductivity relationship, as concentrations obey direct relationship.
The feed solution of Sodium Arsenite (NaAsO2) (As (III)) and Sodium Arsenate (Na2HAsO4) (As V) of 0.5 mg/L were prepared by dissolving in distilled water. The fresh solution was used for performance to avoid the oxidation of As (III) to As (V).
Mode of analysis and equations
The rejection performance (%R) is calculated from usual relationship:
Where is the rejection in percentage, and are the concentration for permeate and the feed solution.
The flux is calculated from the relation:
Where indicates the volume of permeate (litre), t the permeation time (hour) and (m2) the membrane area.
Analytical instruments
FTIR-ATR (Agilent technologies, carry 600 series) spectroscopic analysis was carried out on in the optical range of 400-4000cm-1. The contact angle of water on membrane surface was measured by contact angle meter (DSA 100, Kruss, Germany) at room temperature. 4mL deionized water was added on the samples of the probe and images were captured immediately. The contact angle average was taken for three readings. The topographical measurement was performed using SEM instrument (JSM-7100 F, Russia). Prior to morphology studies membrane samples were washed with deionized water and dried at 50°C overnight. The membrane samples were taken on stub and gold coating was performed on them. Surface charge of membrane was analysed by electro kinetic analysis (Zeta-CAD), France (version 1.04). Ion chromatography (Thermo scientific, DIONEX ICS-5000+ DC) was used for detection of arsenic ion concentration. Conductivity meter (Eutech Instruments, CON 700 (conductivity-TDS meter, Singapore) was used to measure conductivities possessed by sodium chloride solution.
Polysulfone membranes are formed by wet-phase separation technique. It is formed in N,N dimethyl formamide/polysulfone/water system. The preparation of polysulfone membranes by a wet phase inversion technique consists of two main parts: casting of the polymer solution on nonwoven polyester support, immediate immersion of casted polymer in to the water coagulation bath. When polysulfone in casting form contacts with non-solvent water, it immediately forms skin layer. Phase inversion as well as skin layer formation depends upon the surface tension gradients of the solvent and nonsolvent.11 The continuous polymer network is formed because of phase transition state (liquid-liquid demixing and gelation/solidification) in ternary polymer solutions.12,13
Polyamide network are formed through interfacial polymerization of 1,3-phenylene diamine (MPD) and 1,3,5 trimesoyl-chloride (TMC) on the surface of the prepared asymmetric polysulfone membranes. The reaction occurred in hexane phase as high unfavourable partition co-efficient of 1,3,5 trimesoyl-chloride (TMC) makes it unavailable in aqueous medium.14,15 The schematic presentation of thin film composite membrane along with the reaction pathways are earlier presented (Scheme 1). The unreacted -COOH groups develop the charge on the membrane.
ATR-FTIR spectra (Figure 2) analysis shows that strong reflectance at 1489-1630cm-1 is related to the phenyl nucleus stretching mode. The main characteristic absorption band from -SO2- of sulfone groups comes at 1151-1170cm-1. The (-C-O-C-) stretching mode at 1244 and 1323cm-1 are present in the polysulfone spectrum. The characteristic C-N stretching at 1543cm-1 and peak at 789cm-1 showed the presence of polyamide (cross-linked) structure of thin film composite membrane. The 1651cm-1 peak is also observed because of >C=O stretching polyamide structure in the thin film composite membrane spectrum.
The contact angle study shows that water is tried to retain its structure as droplets, rather than spreading over hydrophobic Polysulfone membrane surface. The measurement of contact angles shows that Polysulfone is having contact angle 74.25º. With the interfacial polymerization of 1,3-phenylene diamine and 1,3,5 trimesoyl chloride crosslinked polyamide structure formed and results hydrophilic (contact angle 64.5º) surface of thin-film composite membrane.
Figure 3A & 3B shows the topographical view of polysulfone and thin film composite membrane. The surface morphology of polysulfone membrane shows that pores are distributed in similar fashion throughout the surface. On the other hand granule shaped polyamide is homogenously distributed on thin film composite membrane surface. It results rough patches which reflects distinct difference compared to polysulfone membrane surface.

The zeta potential behaviour of thin film composite membrane surface reflected due to the dissociation of specific ionisable groups on the polymer membrane surface. The zeta potential of thin film composite is experimented and observed -22.14mv. The membrane charge functionality influences polymer network pore structure and Donnan equilibrium, which govern water and salt transport through thin film composite membranes.16 It is because of Donnan potential counter ions (Na+ of salts) draws near towards the membrane where as co-ions repulsion as well as rejection takes place. Higher Donnan potential reflects higher salt rejection of the membrane.17–19 The rejection performance of thin film composite membrane for NaCl and Na2SO4 are in ensemble (Table 1).
Feed conc. 2000mg/L press. Applied : 1.34Mpa |
Flux (LMH) |
Rejection (%) |
NaCl |
32.33 |
94.29 |
Na2SO4 |
31.3 |
96.2 |
Table 1 Salt separation performance of thin film composite membrane
The membrane separation of ionic species is controlled by charge and pore size of the membranes. The arsenic removal through polyamide thin film composite membrane studies involved experiments of As(III) and As(V) in two forms. It is already seen that Arsenic (V) is in common high oxidation state. Table 2 shows that membranes have the potential to separate>90% for As(V). The high rejection of As(V) species are due to relatively large molecular weight of disodium Arsenate (Na2HAsO4) (As(V)) compared to ((NaAsO2) (As (III)) and (107g/gmole and 140g/g-mole for HAsO42-).
Feed conc. 0.5mg/L press. Applied : 1.34Mpa |
Flux (LMH) |
Rejection (%) |
Arsenic (III) |
36.24 |
78.65 |
Arsenic (V) |
36.53 |
92.72 |
Table 2 Thin film composite membrane separation performance of As(III) and As(V) from water
As Sodium Arsenate (Na2HAsO4) exists in divalent form HAsO42-, the rejection is ~14% more compared to NaAsO2 for the low pressure thin film composite membrane. It develops charge due to unreacted acid chloride functional groups and shows negative zeta potential (-22.14mv) in its behavior. The rejection mechanism is same as discussed earlier.
Thin film polyamide composite membranes were prepared through interfacial polymerization 1,3 phenylene diamine (in water) with 1,3,5 trimesoyl chloride (in hexane). It shows salt rejection property. The present study demonstrates that the tailor-made thin film composite membrane has the potential to remove Arsenic from water. The membranes showed its potential to remove ~92.72%As(V) from water. It also shows that As(V) removal is much more (~14%) compared to As(III).
The Registered number of the work is CSIR-CSMCRI-005/2018. Authors are grateful to Analytical and Environmental Science Division and Centralized Instrument Facility of the Institute.
The author declares no conflict of interest.

©2018 Mehta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.